Small molecule selectively suppresses MYC transcription in cancer cells
- PMID: 28292893
- PMCID: PMC5380033
- DOI: 10.1073/pnas.1702663114
Small molecule selectively suppresses MYC transcription in cancer cells
Abstract
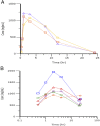
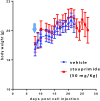
Stauprimide is a staurosporine analog that promotes embryonic stem cell (ESC) differentiation by inhibiting nuclear localization of the MYC transcription factor NME2, which in turn results in down-regulation of MYC transcription. Given the critical role the oncogene MYC plays in tumor initiation and maintenance, we explored the potential of stauprimide as an anticancer agent. Here we report that stauprimide suppresses MYC transcription in cancer cell lines derived from distinct tissues. Using renal cancer cells, we confirmed that stauprimide inhibits NME2 nuclear localization. Gene expression analysis also confirmed the selective down-regulation of MYC target genes by stauprimide. Consistent with this activity, administration of stauprimide inhibited tumor growth in rodent xenograft models. Our study provides a unique strategy for selectively targeting MYC transcription by pharmacological means as a potential treatment for MYC-dependent tumors.
Keywords: MYC; NME2; cancer; nuclear localization; stauprimide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Dang CV, O’donnell KA, Juopperi T. The great MYC escape in tumorigenesis. Cancer Cell. 2005;8(3):177–178. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

