Plaques Formed by Mutagenized Viral Populations Have Elevated Coinfection Frequencies
- PMID: 28292984
- PMCID: PMC5350468
- DOI: 10.1128/mBio.02020-16
Plaques Formed by Mutagenized Viral Populations Have Elevated Coinfection Frequencies
Abstract
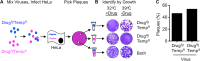
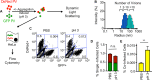
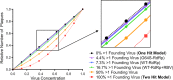
The plaque assay is a common technique used to measure virus concentrations and is based upon the principle that each plaque represents a single infectious unit. As such, the number of plaques is expected to correlate linearly with the virus dilution plated, and each plaque should be formed by a single founder virus. Here, we examined whether more than one virus can contribute to plaque formation. By using genetic and phenotypic assays with genetically marked polioviruses, we found that multiple parental viruses are present in 5 to 7% of plaques, even at an extremely low multiplicity of infection. We demonstrated through visual and biophysical assays that, like many viral stocks, our viral stocks contain both single particles and aggregates. These data suggest that aggregated virions are capable of inducing coinfection and chimeric plaque formation. In fact, inducing virion aggregation via exposure to low pH increased coinfection in a flow cytometry-based assay. We hypothesized that plaques generated by viruses with high mutation loads may have higher coinfection frequencies due to processes restoring fitness, such as complementation and recombination. Indeed, we found that coinfection frequency correlated with mutation load, with 17% chimeric plaque formation for heavily mutagenized viruses. Importantly, the frequency of chimeric plaques may be underestimated by up to threefold, since coinfection with the same parental virus cannot be scored in our assay. This work indicates that more than one virus can contribute to plaque formation and that coinfection may assist plaque formation in situations where the amount of genome damage is high.IMPORTANCE One of the most common methods to quantify viruses is the plaque assay, where it is generally presumed that each plaque represents a single infectious virus. Using genetically marked polioviruses, we demonstrate that a plaque can contain more than one parental virus, likely due to aggregates within virus stocks that induce coinfection of a cell. A relatively small number of plaques are the products of coinfection for our standard virus stocks. However, mutagenized virus stocks with increased genome damage give rise to a higher amount of plaques that are chimeric. These results suggest that coinfection may aid plaque formation of viruses with genome damage, possibly due to processes such as complementation and recombination. Overall, our results suggest that the relationship between viral dilution and plaque number may not be linear, particularly for mutagenized viral populations.
Keywords: coinfection; evolution; mutagen; poliovirus.
Copyright © 2017 Aguilera et al.
Figures



Comment in
-
Observed High Coinfection Rates Seem To Be a Result of Overlapping Plaques.mBio. 2017 Aug 8;8(4):e01000-17. doi: 10.1128/mBio.01000-17. mBio. 2017. PMID: 28790209 Free PMC article. No abstract available.
-
Reply to Drayman, "Observed High Coinfection Rates Seem To Be a Result of Overlapping Plaques".mBio. 2017 Aug 8;8(4):e01201-17. doi: 10.1128/mBio.01201-17. mBio. 2017. PMID: 28790211 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

