Planar cell polarity in development and disease
- PMID: 28293032
- PMCID: PMC5826606
- DOI: 10.1038/nrm.2017.11
Planar cell polarity in development and disease
Abstract
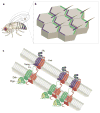
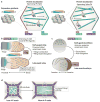
Planar cell polarity (PCP) is an essential feature of animal tissues, whereby distinct polarity is established within the plane of a cell sheet. Tissue-wide establishment of PCP is driven by multiple global cues, including gradients of gene expression, gradients of secreted WNT ligands and anisotropic tissue strain. These cues guide the dynamic, subcellular enrichment of PCP proteins, which can self-assemble into mutually exclusive complexes at opposite sides of a cell. Endocytosis, endosomal trafficking and degradation dynamics of PCP components further regulate planar tissue patterning. This polarization propagates throughout the whole tissue, providing a polarity axis that governs collective morphogenetic events such as the orientation of subcellular structures and cell rearrangements. Reflecting the necessity of polarized cellular behaviours for proper development and function of diverse organs, defects in PCP have been implicated in human pathologies, most notably in severe birth defects.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

