Paclitaxel-loaded star-shaped copolymer nanoparticles for enhanced malignant melanoma chemotherapy against multidrug resistance
- PMID: 28293102
- PMCID: PMC5345981
- DOI: 10.2147/DDDT.S127328
Paclitaxel-loaded star-shaped copolymer nanoparticles for enhanced malignant melanoma chemotherapy against multidrug resistance
Abstract
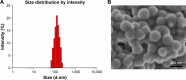
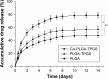
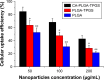
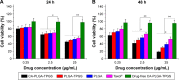
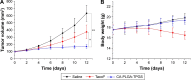
Malignant melanoma (MM) is the most dangerous type of skin cancer with annually increasing incidence and death rates. However, chemotherapy for MM is restricted by low topical drug concentration and multidrug resistance. In order to surmount the limitation and to enhance the therapeutic effect on MM, a new nanoformulation of paclitaxel (PTX)-loaded cholic acid (CA)-functionalized star-shaped poly(lactide-co-glycolide) (PLGA)-D-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) nanoparticles (NPs) (shortly PTX-loaded CA-PLGA-TPGS NPs) was fabricated by a modified method of nanoprecipitation. The particle size, zeta potential, morphology, drug release profile, drug encapsulation efficiency, and loading content of PTX-loaded NPs were detected. As shown by confocal laser scanning, NPs loaded with coumarin-6 were internalized by human melanoma cell line A875. The cellular uptake efficiency of CA-PLGA-TPGS NPs was higher than those of PLGA NPs and PLGA-TPGS NPs. The antitumor effects of PTX-loaded NPs were evaluated by the MTT assay in vitro and by a xenograft tumor model in vivo, demonstrating that star-shaped PTX-loaded CA-PLGA-TPGS NPs were significantly superior to commercial PTX formulation Taxol®. Such drug delivery nanocarriers are potentially applicable to the improvement of clinical MM therapy.
Keywords: drug delivery; enhanced therapeutic effects; malignant melanoma; nanoparticles; paclitaxel.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures



References
-
- Zhang W, Shi Y, Chen Y, Hao J, Sha X, Fang X. The potential of Pluronic polymeric micelles encapsulated with paclitaxel for the treatment of melanoma using subcutaneous and pulmonary metastatic mice models. Biomaterials. 2011;32(25):5934–5944. - PubMed
-
- Ruttala HB, Ko YT. Liposomal co-delivery of curcumin and albumin/paclitaxel nanoparticle for enhanced synergistic antitumor efficacy. Colloids Surf B Biointerfaces. 2015;128:419–426. - PubMed
-
- Kannan V, Balabathula P, Thoma LA, Wood GC. Effect of sucrose as a lyoprotectant on the integrity of paclitaxel-loaded liposomes during lyophilization. J Liposome Res. 2015;25(4):270–278. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

