[Pyr1]Apelin-13(1-12) Is a Biologically Active ACE2 Metabolite of the Endogenous Cardiovascular Peptide [Pyr1]Apelin-13
- PMID: 28293165
- PMCID: PMC5329011
- DOI: 10.3389/fnins.2017.00092
[Pyr1]Apelin-13(1-12) Is a Biologically Active ACE2 Metabolite of the Endogenous Cardiovascular Peptide [Pyr1]Apelin-13
Abstract
Aims: Apelin is a predicted substrate for ACE2, a novel therapeutic target. Our aim was to demonstrate the endogenous presence of the putative ACE2 product [Pyr1]apelin-13(1-12) in human cardiovascular tissues and to confirm it retains significant biological activity for the apelin receptor in vitro and in vivo. The minimum active apelin fragment was also investigated. Methods and Results: [Pyr1]apelin-13 incubated with recombinant human ACE2 resulted in de novo generation of [Pyr1]apelin-13(1-12) identified by mass spectrometry. Endogenous [Pyr1]apelin-13(1-12) was detected by immunostaining in human heart and lung localized to the endothelium. Expression was undetectable in lung from patients with pulmonary arterial hypertension. In human heart [Pyr1]apelin-13(1-12) (pKi = 8.04 ± 0.06) and apelin-13(F13A) (pKi = 8.07 ± 0.24) competed with [125I]apelin-13 binding with nanomolar affinity, 4-fold lower than for [Pyr1]apelin-13 (pKi = 8.83 ± 0.06) whereas apelin-17 exhibited highest affinity (pKi = 9.63 ± 0.17). The rank order of potency of peptides to inhibit forskolin-stimulated cAMP was apelin-17 (pD2 = 10.31 ± 0.28) > [Pyr1]apelin-13 (pD2 = 9.67 ± 0.04) ≥ apelin-13(F13A) (pD2 = 9.54 ± 0.05) > [Pyr1]apelin-13(1-12) (pD2 = 9.30 ± 0.06). The truncated peptide apelin-13(R10M) retained nanomolar potency (pD2 = 8.70 ± 0.04) but shorter fragments exhibited low micromolar potency. In a β-arrestin recruitment assay the rank order of potency was apelin-17 (pD2 = 10.26 ± 0.09) >> [Pyr1]apelin-13 (pD2 = 8.43 ± 0.08) > apelin-13(R10M) (pD2 = 8.26 ± 0.17) > apelin-13(F13A) (pD2 = 7.98 ± 0.04) ≥ [Pyr1]apelin-13(1-12) (pD2 = 7.84 ± 0.06) >> shorter fragments (pD2 < 6). [Pyr1]apelin-13(1-12) and apelin-13(F13A) contracted human saphenous vein with similar sub-nanomolar potencies and [Pyr1]apelin-13(1-12) was a potent inotrope in paced mouse right ventricle and human atria. [Pyr1]apelin-13(1-12) elicited a dose-dependent decrease in blood pressure in anesthetized rat and dose-dependent increase in forearm blood flow in human volunteers. Conclusions: We provide evidence that ACE2 cleaves [Pyr1]apelin-13 to [Pyr1]apelin-13(1-12) and this cleavage product is expressed in human cardiovascular tissues. We have demonstrated biological activity of [Pyr1]apelin-13(1-12) at the human and rodent apelin receptor in vitro and in vivo. Our data show that reported enhanced ACE2 activity in cardiovascular disease should not significantly compromise the beneficial effects of apelin based therapies for example in PAH.
Keywords: ACE2; [Pyr1]apelin-13(1–12); apelin; apelin receptor; biased signaling; forearm plethysmography; human heart; pulmonary arterial hypertension.
Figures

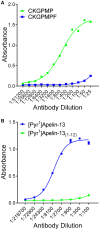
 , corresponding to [Pyr1]Apelin-13(1–12)) relative to CKGPMPF (
, corresponding to [Pyr1]Apelin-13(1–12)) relative to CKGPMPF ( , corresponding to [Pyr1]Apelin-13). (B) The apelin-12 antiserum demonstrated selectivity to [Pyr1]Apelin-13 (
, corresponding to [Pyr1]Apelin-13). (B) The apelin-12 antiserum demonstrated selectivity to [Pyr1]Apelin-13 ( ) relative to [Pyr1]Apelin-13(1–12) (
) relative to [Pyr1]Apelin-13(1–12) ( ).
).

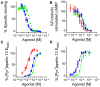
 ) and [Pyr1]apelin-13(1–12) (
) and [Pyr1]apelin-13(1–12) ( ) in human left ventricle (n = 3). In cell based assays [Pyr1]apelin-13 (
) in human left ventricle (n = 3). In cell based assays [Pyr1]apelin-13 ( ), [Pyr1]apelin-13(1–12) (
), [Pyr1]apelin-13(1–12) ( ), and apelin-17 (
), and apelin-17 ( ) (B) inhibited forskolin-stimulated cAMP production; (C) induced β-arrestin recruitment and (D) triggered apelin receptor internalization.
) (B) inhibited forskolin-stimulated cAMP production; (C) induced β-arrestin recruitment and (D) triggered apelin receptor internalization.
 , n = 9) and apelin-13(F13A) (
, n = 9) and apelin-13(F13A) ( , n = 9) in endothelium-denuded human saphenous vein in vitro. (B) Inotropic responses to (B) [Pyr1]apelin-13(1–12) (n = 6) and (C) isoprenaline (n = 4) in mouse paced right ventricle.
, n = 9) in endothelium-denuded human saphenous vein in vitro. (B) Inotropic responses to (B) [Pyr1]apelin-13(1–12) (n = 6) and (C) isoprenaline (n = 4) in mouse paced right ventricle.
 , n = 5) resulted in a significant dose-dependent decrease in blood pressure in rat in vivo. Other parameters (B) stroke volume (SV), (C) cardiac output (CO), (D) peak velocity (PV) and velocity-time interval (VTI) were unaffected at any dose of [Pyr1]apelin-13(1–12). Significantly different from baseline; *P < 0.05, **P < 0.01, ***P < 0.001. One-way ANOVA repeated measures compared to baseline with Dunnett's multiple comparison test.
, n = 5) resulted in a significant dose-dependent decrease in blood pressure in rat in vivo. Other parameters (B) stroke volume (SV), (C) cardiac output (CO), (D) peak velocity (PV) and velocity-time interval (VTI) were unaffected at any dose of [Pyr1]apelin-13(1–12). Significantly different from baseline; *P < 0.05, **P < 0.01, ***P < 0.001. One-way ANOVA repeated measures compared to baseline with Dunnett's multiple comparison test.
 , n = 12) resulted in a dose-dependent increase in forearm blood flow. Significantly different from baseline; *P < 0.05, **P < 0.01. One-way ANOVA repeated measures compared to baseline with Dunnett's multiple comparison test.
, n = 12) resulted in a dose-dependent increase in forearm blood flow. Significantly different from baseline; *P < 0.05, **P < 0.01. One-way ANOVA repeated measures compared to baseline with Dunnett's multiple comparison test.
References
-
- Barnes G. D., Alam S., Carter G., Pedersen C. M., Lee K. M., Hubbard T. J., et al. . (2013). Sustained cardiovascular actions of APJ agonism during renin-angiotensin system activation and in patients with heart failure. Circ. Heart Fail. 6, 482–491. 10.1161/CIRCHEARTFAILURE.111.000077 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

