Prevalence of M75 Streptococcus pyogenes Strains Harboring slaA Gene in Patients Affected by Pediatric Obstructive Sleep Apnea Syndrome in Central Italy
- PMID: 28293224
- PMCID: PMC5329643
- DOI: 10.3389/fmicb.2017.00294
Prevalence of M75 Streptococcus pyogenes Strains Harboring slaA Gene in Patients Affected by Pediatric Obstructive Sleep Apnea Syndrome in Central Italy
Abstract
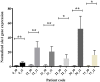
Recently we reported an association between pediatric obstructive sleep apnea syndrome (OSAS) and Group A streptococcus (GAS) sub-acute chronic tonsil colonization. We showed that GAS may contribute to tonsil hyperplasia via a streptolysin O (SLO)-dependent cysteinyl leukotrienes (CysLTs) production, which can trigger T and B cell proliferation. In the present study, we characterized the GAS strains isolated from pediatric OSAS patients in comparison with a panel of age and sex matched GAS strains unrelated to OSAS, but isolated in the same area and during the same period ranging from 2009 to 2013. We found that slaA gene, previously reported to be associated to CysLTs production pathway, was significantly associated to GAS OSAS strains. Moreover, the most numerous group (32%) of the GAS OSAS strains belonged to M75 type, and 6 out of 7 of these strains harbored the slaA gene. Multilocus Sequence Typing (MLST) experiments demonstrated that the clone emm75/ST49/ smeZ, slaA was associated to OSAS cases. In conclusion, we found an association between slaA gene and the GAS OSAS strains, and we showed that the clone emm75/ST49 harboring genes smeZ and slaA was exclusively isolated from patients affected by OSAS, thus suggesting that this genotype might be associated to the pathogenesis of OSAS, although further studies are needed to elucidate the possible role of SlaA in tonsil hypertrophy development.
Keywords: Multilocus Sequence Typing (MLST); Streptococcus pyogenes; molecular epidemiology; obstructive sleep apnea syndrome (OSAS); slaA gene.
Figures
References
-
- American Thoracic Society (1996). Standards and indications for cardiopulmonary sleep studies in children. Am. J. Respir. Crit. Care Med. 153, 866–878. - PubMed
-
- Ardanuy C., Domenech A., Rolo D., Calatayud L., Tubau F., Ayats J., et al. . (2010). Molecular characterization of macrolide- and multidrug-resistant Streptococcus pyogenes isolated from adult patients in Barcelona, Spain (1993–2008). J. Antimicrob. Chemother. 65, 634–643. 10.1093/jac/dkq006 - DOI - PubMed
-
- Bao Y. J., Liang Z., Mayfield J. A., Donahue D. L., Carothers K. E., Lee S. W., et al. . (2016). Genomic characterization of a pattern D Streptococcus pyogenes emm53 isolate reveals a genetic rationale for invasive skin tropicity. J. Bacteriol. 198, 1712–1724. 10.1128/JB.01019-15 - DOI - PMC - PubMed
-
- Beres S. B., Sylva G. L., Barbian K. D., Lei B., Hoff J. S., Mammarella N. D., et al. . (2002). Genome sequence of a serotype M3 strain of group a Streptococcus: phage-encoded toxins, the high-virulence phenotype, and clone emergence. Proc. Natl. Acad. Sci. U.S.A. 99, 10078–10083. 10.1073/pnas.152298499 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

