MLKL Mediated Necroptosis Accelerates JEV-Induced Neuroinflammation in Mice
- PMID: 28293227
- PMCID: PMC5328978
- DOI: 10.3389/fmicb.2017.00303
MLKL Mediated Necroptosis Accelerates JEV-Induced Neuroinflammation in Mice
Abstract
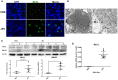
Japanese encephalitis virus (JEV) is the most prevalent cause of viral encephalitis in Asia and the western Pacific. Neuronal death caused by JEV infection and inflammation induced cytotoxicity leads to progression and deterioration of Japanese encephalitis (JE). Mixed-lineage kinase domain-like protein (MLKL) mediated necroptosis is a newly discovered pathway of programmed cell death and participates in many inflammatory diseases. In this study, we demonstrated for the first time that necroptosis was involved in the neuronal loss during JE via immune-electron microscopy and immunochemistry. The expression of MLKL in neurons was upregulated in presence of JEV infection in vitro and in vivo. Deletion of MLKL alleviated the progression of JE and decreased the level of inflammatory cytokines in mice model. Taken together, this study provides evidence for the participation of necroptosis in the pathogenesis of JEV infection.
Keywords: Japanese encephalitis virus; MLKL; inflammation; necroptosis; neuronal death.
Figures





References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

