Dysbiosis of Gut Microbiota Associated with Clinical Parameters in Polycystic Ovary Syndrome
- PMID: 28293234
- PMCID: PMC5328957
- DOI: 10.3389/fmicb.2017.00324
Dysbiosis of Gut Microbiota Associated with Clinical Parameters in Polycystic Ovary Syndrome
Abstract
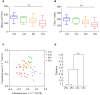
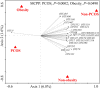
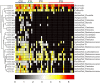
Polycystic ovary syndrome (PCOS) is a common endocrine and metabolic disorder in women. Gut microbiota has been implicated to play a critical role in metabolic diseases and may modulate the secretion of mediators of the brain-gut axis. Interaction between gut microbiota and the endocrine and biochemical disturbances in PCOS still remains elusive. Here, we showed an altered gut microbiota significantly correlated with PCOS phenotype. There were 33 patients with PCOS (non-obese PCOS individuals, PN, n = 12; obese PCOS individuals, PO, n = 21) as well as 15 control subjects (non-obese control individuals, CN, n = 9; obese control individuals, CO, n = 6) enrolled in our study. The plasma levels of serotonin, ghrelin, and peptide YY (PYY) were significantly decreased in patients with PCOS compared with controls, and have a significantly negative correlation with waist circumference and testosterone. Sequencing of the V3-V4 region of the 16S rRNA gene in fecal samples revealed the substantial differences of gut microbial species between the PCOS and non-obese controls. Bacterial species were clustered into 23 co-abundance groups (CAGs) based on the SparCC correlation coefficients of their relative abundance. The CAGs increased in PCOS, including the bacteria belonging to Bacteroides, Escherichia/Shigella and Streptococcus, were negatively correlated with ghrelin, and positively correlated with testosterone and BMI. Furthermore, the CAGs that were decreased in PCOS, including the bacteria from Akkermansia and Ruminococcaceae, showed opposite relationship with body-weight, sex-hormone, and brain-gut peptides. In conclusion, gut microbial dysbiosis in women with PCOS is associated with the disease phenotypes.
Keywords: ghrelin; gut microbiota; obesity; polycystic ovary syndrome; serotonin; testosterone.
Figures


References
-
- Anderson M. (2004). CAP: A FORTRAN Computer Program for Canonical Analysis of Principal Coordinates. Auckland: University of Auckland; 14.
-
- Behboudigandevani S., Tehrani F. R., Dovom M. R., Farahmand M., Khomami M. B., Noroozzadeh M., et al. (2016). Insulin resistance in obesity and polycystic ovary syndrome: systematic review and meta-analysis of observational studies. Gynecol. Endocrinol. 32 1–11. 10.3109/09513590.2015.1117069 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

