Evaluation of the irritable bowel syndrome severity index in Japanese male patients with irritable bowel syndrome with diarrhea
- PMID: 28293280
- PMCID: PMC5346243
- DOI: 10.1186/s13030-017-0092-x
Evaluation of the irritable bowel syndrome severity index in Japanese male patients with irritable bowel syndrome with diarrhea
Abstract
Background: Previous studies have indicated that ramosetron, a 5-hydroxytryptamine-3 receptor antagonist, achieves global improvement in irritable bowel syndrome (IBS) symptoms in male patients with IBS with diarrhea (IBS-D). However, in addition to global assessment it was deemed important to assess "clinically meaningful improvements, focusing on the patient's chief complaint and the severity of major IBS symptoms". We performed a randomized, placebo-controlled, phase IV pilot study to explore and examine efficacy variables that allow such evaluation of ramosetron in male patients with IBS-D.
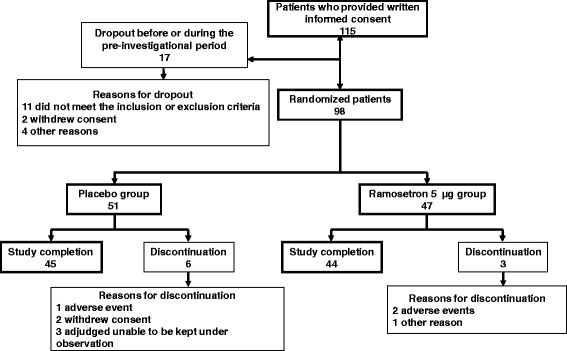
Methods: We performed a prospective study of 115 male outpatients with IBS-D (according to the Rome III criteria), from June 2009 to December 2009 at 25 centers in Japan. After a one-week baseline period, subjects received either 5 μg of ramosetron (n = 47) or placebo (n = 51) once daily for 12 weeks. To evaluate "clinically meaningful improvements focusing on the severity of major IBS symptoms," the Japanese version of the IBS severity index (IBSSI-J) was used.
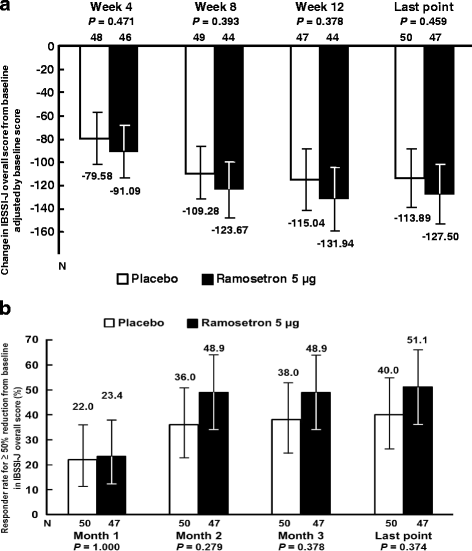
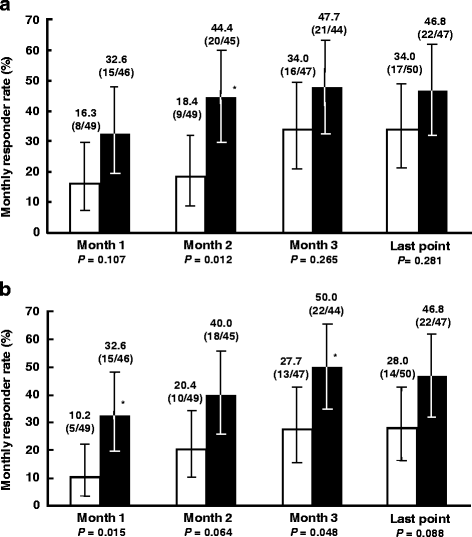
Results: Change in IBSSI-J overall score from baseline was -133.5 ± 110.72 in the ramosetron 5 μg group and -108.2 ± 94.44 in the placebo group (P = 0.228) at the last evaluation point. Differences in responder rates for at least a 50% reduction from baseline in IBSSI-J between the ramosetron 5 μg group and the placebo group were over 10%, except Month 1. The monthly responder rate for global assessment of relief of overall IBS symptoms in the ramosetron 5 μg group showed a statistically significant improvement compared to placebo at the second month (44.4% vs 18.4%, P = 0.012). The proportion of patients who had a ≥ 50% reduction in IBSSI-J overall score was 24/37 (64.9%) in the responder group on global assessment and 18/54 (33.3%) in the non-responder group at Week 12.
Conclusions: Further examination will be needed before IBSSI-J can be used in clinical trials of agents for IBS-D. However, this study revealed that response on global assessment was correlated with improvement in the IBSSI-J, suggesting that global assessment reflects improvement of the symptom severity of patients with IBS-D. (Clinicaltrials.gov ID: NCT00918411 Registered 9 June 2009).
Keywords: 5-hydroxytryptamine (5-HT); Abdominal discomfort; Abdominal pain; Global improvement; Irritable bowel syndrome severity index; Stool consistency.
Figures

References
-
- Miyata K, Ito H, Fukudo S. Involvement of the 5-HT3 receptor in CRH-induced defecation in rats. Am J Physiol. 1998;274:G827–G831. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

