Microvascular Fragment Transplantation Improves Rat Dorsal Skin Flap Survival
- PMID: 28293502
- PMCID: PMC5222647
- DOI: 10.1097/GOX.0000000000001140
Microvascular Fragment Transplantation Improves Rat Dorsal Skin Flap Survival
Abstract
Background: The development of flap necrosis distally remains a concern during microsurgical flap transfers because, at least in part, of decreased perfusion. Microvascular fragments (MVFs) are microvessels isolated from adipose tissue that are capable of improving tissue perfusion in a variety of tissue defects. The aim of this study was to determine whether the transplantation of MVFs in a dorsal rat skin flap model can improve flap survival.
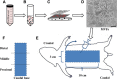
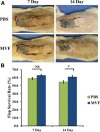
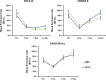
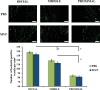
Methods: A 10 × 3 cm flap was raised in a cranial to caudal fashion on the dorsal side of 16 Lewis rats, with the caudal side remaining intact. The rats were equally divided into a treatment group (MVFs) and a control group (sterile saline). At the time of surgery, sterile saline with or without MVFs was injected directly into the flap. Microvessel density was determined after harvesting flap tissue by counting vessels that positively stained for Griffonia simplicifolia lectin I-isolectin B4. Laser Doppler was used to measure blood flow before and after surgery and 7 and 14 days later. Flap survival was evaluated 7 and 14 days after surgery by evaluating the percentage of viable tissue of the flap with photodigital planimetry.
Results: Despite the lack of a significant difference in microvessel density and tissue perfusion, flap survival increased 6.4% (P < 0.05) in MVF-treated animals compared with controls.
Conclusions: The use of MVFs may be a means to improve flap survival. Future studies are required to delineate mechanisms whereby this occurs and to further optimize their application.
Figures
References
-
- Maciel-Miranda A, Morris SF, Hallock GG. Local flaps, including pedicled perforator flaps: anatomy, technique, and applications. Plast Reconstr Surg. 2013;131:896e–911e. - PubMed
-
- Lineaweaver W, Akdemir O, Schleich A. Management strategies following microsurgical flap failure. Microsurgery. 2010;30:61–63. - PubMed
-
- Roehl KR, Mahabir RC. A practical guide to free tissue transfer. Plast Reconstr Surg. 2013;132:147e–158e. - PubMed
-
- Chen KT, Mardini S, Chuang DC, et al. Timing of presentation of the first signs of vascular compromise dictates the salvage outcome of free flap transfers. Plast Reconstr Surg. 2007;120:187–195. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
