Induction of Atypical Autophagy by Porcine Hemagglutinating Encephalomyelitis Virus Contributes to Viral Replication
- PMID: 28293544
- PMCID: PMC5328988
- DOI: 10.3389/fcimb.2017.00056
Induction of Atypical Autophagy by Porcine Hemagglutinating Encephalomyelitis Virus Contributes to Viral Replication
Abstract
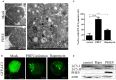
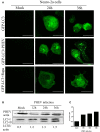
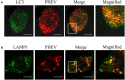
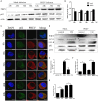
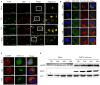
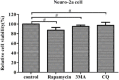
Autophagy is a basic biological metabolic process involving in intracellular membrane transport pathways that recycle cellular components and eliminate intracellular microorganisms within the lysosome. Autophagy also plays an important part in virus infection and propagation. However, some pathogens, including viruses, have evolved unique trick to escape or exploit autophagy. This study explores the mechanism of autophagy induction by porcine hemagglutinating encephalomyelitis virus (PHEV) in Neuro-2a cells, and examines the role of autophagy in PHEV replication. PHEV triggered autophagy in Neuro-2a cells is dependent on the presence of bulk double- or single-membrane vacuoles, the accumulation of GFP-LC3 fluorescent dots, and the LC3 lipidation. In addition, PHEV induced an incomplete autophagic effect because the degradation level of p62 did not change in PHEV-infected cells. Further validation was captured using LysoTracker and lysosome-associated membrane protein by indirect immunofluorescence labeling in PHEV-infected cells. We also investigated the change in viral replication by pharmacological experiments with the autophagy inducer rapamycin or the autophagy inhibitor 3-MA, and the lysosomal inhibitor chloroquine (CQ). Suppression of autophagy by 3-MA increased viral replication, compared with the mock treatment, while promoting of autophagy by rapamycin reduced PHEV replication. CQ treatment enhanced the LC3 lipidation in PHEV-infected Neuro-2a cells but lowered PHEV replication. These results show that PHEV infection induces atypical autophagy and causes the appearance of autophagosomes but blocks the fusion with lysosomes, which is necessary for the replication of PHEV in nerve cells.
Keywords: LC3; atypical autophagy; autophagic flux; neuro-2a cells; neurotropic virus; porcine hemagglutinating encephalomyelitis virus; virus replication.
Figures

References
-
- Andries K., Pensaert M. B. (1980). Immunofluorescence studies on the pathogenesis of hemagglutinating encephalomyelitis virus infection in pigs after oronasal inoculation. Am. J. Vet. Res. 41, 1372–1378. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

