Urinary Biomarker Panel to Improve Accuracy in Predicting Prostate Biopsy Result in Chinese Men with PSA 4-10 ng/mL
- PMID: 28293631
- PMCID: PMC5331314
- DOI: 10.1155/2017/2512536
Urinary Biomarker Panel to Improve Accuracy in Predicting Prostate Biopsy Result in Chinese Men with PSA 4-10 ng/mL
Abstract
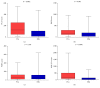
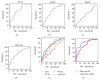
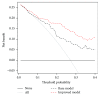
This study aims to evaluate the effectiveness and clinical performance of a panel of urinary biomarkers to diagnose prostate cancer (PCa) in Chinese men with PSA levels between 4 and 10 ng/mL. A total of 122 patients with PSA levels between 4 and 10 ng/mL who underwent consecutive prostate biopsy at three hospitals in China were recruited. First-catch urine samples were collected after an attentive prostate massage. Urinary mRNA levels were measured by quantitative real-time polymerase chain reaction (qRT-PCR). The predictive accuracy of these biomarkers and prediction models was assessed by the area under the curve (AUC) of the receiver-operating characteristic (ROC) curve. The diagnostic accuracy of PCA3, PSGR, and MALAT-1 was superior to that of PSA. PCA3 performed best, with an AUC of 0.734 (95% CI: 0.641, 0.828) followed by MALAT-1 with an AUC of 0.727 (95% CI: 0.625, 0.829) and PSGR with an AUC of 0.666 (95% CI: 0.575, 0.749). The diagnostic panel with age, prostate volume, % fPSA, PCA3 score, PSGR score, and MALAT-1 score yielded an AUC of 0.857 (95% CI: 0.780, 0.933). At a threshold probability of 20%, 47.2% of unnecessary biopsies may be avoided whereas only 6.2% of PCa cases may be missed. This urinary panel may improve the current diagnostic modality in Chinese men with PSA levels between 4 and 10 ng/mL.
Conflict of interest statement
The authors declare that there are no competing financial interests.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

