Growth Hormone Receptor Knockdown Sensitizes Human Melanoma Cells to Chemotherapy by Attenuating Expression of ABC Drug Efflux Pumps
- PMID: 28293855
- PMCID: PMC10355985
- DOI: 10.1007/s12672-017-0292-7
Growth Hormone Receptor Knockdown Sensitizes Human Melanoma Cells to Chemotherapy by Attenuating Expression of ABC Drug Efflux Pumps
Abstract
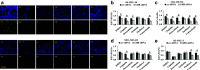
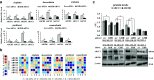
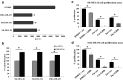
Melanoma remains one of the most therapy-resistant forms of human cancer despite recent introductions of highly efficacious targeted therapies. The intrinsic therapy resistance of human melanoma is largely due to abundant expression of a repertoire of xenobiotic efflux pumps of the ATP-binding cassette (ABC) transporter family. Here, we report that GH action is a key mediator of chemotherapeutic resistance in human melanoma cells. We investigated multiple ABC efflux pumps (ABCB1, ABCB5, ABCB8, ABCC1, ABCC2, ABCG1, and ABCG2) reportedly associated with melanoma drug resistance in different human melanoma cells and tested the efficacy of five different anti-cancer compounds (cisplatin, doxorubicin, oridonin, paclitaxel, vemurafenib) with decreased GH action. We found that GH treatment of human melanoma cells upregulates expression of multiple ABC transporters and increases the EC50 of melanoma drug vemurafenib. Also, vemurafenib-resistant melanoma cells had upregulated levels of GH receptor (GHR) expression as well as ABC efflux pumps. GHR knockdown (KD) using siRNA in human melanoma cells treated with sub-EC50 doses of anti-tumor compounds resulted in significantly increased drug retention, decreased cell proliferation and increased drug efficacy, compared to mock-transfected controls. Our set of findings identify an unknown mechanism of GH regulation in mediating melanoma drug resistance and validates GHR as a unique therapeutic target for sensitizing highly therapy-resistant human melanoma cells to lower doses of anti-cancer drugs.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

