Navigating the Chiral Pool in the Total Synthesis of Complex Terpene Natural Products
- PMID: 28293944
- PMCID: PMC5638449
- DOI: 10.1021/acs.chemrev.6b00834
Navigating the Chiral Pool in the Total Synthesis of Complex Terpene Natural Products
Abstract
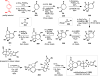
The pool of abundant chiral terpene building blocks (i.e., "chiral pool terpenes") has long served as a starting point for the chemical synthesis of complex natural products, including many terpenes themselves. As inexpensive and versatile starting materials, such compounds continue to influence modern synthetic chemistry. This review highlights 21st century terpene total syntheses which themselves use small, terpene-derived materials as building blocks. An outlook to the future of research in this area is highlighted as well.
Conflict of interest statement
The authors declare no competing financial interests.
Figures















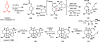











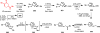





References
-
- Breitmaier E. Terpenes: Flavors, Fragrances, Pharmaca, Pheromones. Wiley-VCH; Weinheim, Germany: 2006.
-
- Rude MA, Schirmer A. New microbial fuels: a biotech perspective. Curr Opin Microbiol. 2009;12:274–281. - PubMed
-
- Wilbon PA, Chu F, Tang C. Progress in Renewable Polymers from Natural Terpenes, Terpenoids, and Rosin. Macromol Rapid Commun. 2013;34:8–37. - PubMed
-
- Blaser H-U. The chiral pool as a source of enantioselective catalysts and auxiliaries. Chem Rev. 1992;92:935–952.
-
- Ager D, editor. Handbook of Chiral Chemicals. 2nd. CRC Press, Taylor & Francis; Boca Raton, FL: 2005.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

