Catalytic, Enantioselective, Intramolecular Sulfenoamination of Alkenes with Anilines
- PMID: 28294614
- PMCID: PMC5388071
- DOI: 10.1021/acs.joc.7b00391
Catalytic, Enantioselective, Intramolecular Sulfenoamination of Alkenes with Anilines
Abstract
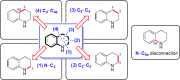
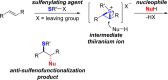
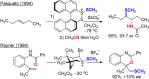
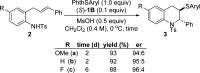
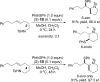
A method for the catalytic, enantioselective, intramolecular sulfenoamination of alkenes with aniline nucleophiles has been developed. The method employs a chiral, Lewis basic selenophosphoramide catalyst and a Brønsted acid co-catalyst to promote stereocontrolled C-N and C-S bond formation by activation of an achiral sulfenylating agent. Benzoannulated nitrogen-containing heterocycles such as indolines, tetrahydroquinolines, and tetrahydrobenzazepines were prepared with high to excellent enantioselectivities. The impact of tether length and electron density of both the nucleophile and olefin on the reactivity, site selectivity, and enantioselectivity were investigated and interpreted in terms of substrate-dependent stereodetermining thiiranium ion formation or capture.
Conflict of interest statement
The authors declare no competing financial interest.
Figures















References
-
- Sridharan V.; Suryavanshi P. A.; Menéndez J. C. Chem. Rev. 2011, 111, 7157–7259. 10.1021/cr100307m. - DOI - PubMed
- Katritzky A.; Rachwal S.; Rachwal B. Tetrahedron 1996, 52, 15031–15070. 10.1016/S0040-4020(96)00911-8. - DOI
- Nammalwar B.; Bunce R. A. Molecules 2014, 19, 204–232. 10.3390/molecules19010204. - DOI - PMC - PubMed
- Muñoz G. D.; Dudley G. B. Org. Prep. Proced. Int. 2015, 47, 179–206. 10.1080/00304948.2015.1025012. - DOI
-
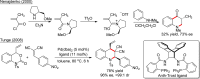
- Rueping M.; Antonchick A. P.; Theissmann T. Angew. Chem., Int. Ed. 2006, 45, 3683–3686. 10.1002/anie.200600191. - DOI - PubMed
- Rueping M.; Theissmann T.; Raja S.; Bats J. W. Adv. Synth. Catal. 2008, 350, 1001–1006. 10.1002/adsc.200800020. - DOI
- Wang D.-S.; Chen Q.-A.; Lu S.-M.; Zhou Y.-G. Chem. Rev. 2012, 112, 2557–2590. 10.1021/cr200328h. - DOI - PubMed
- Cai X.-F.; Chen M.-W.; Ye Z.-S.; Guo R.-N.; Shi L.; Li Y.-Q.; Zhou Y.-G. Chem. - Asian J. 2013, 8, 1381–1385. 10.1002/asia.201300380. - DOI - PubMed
-
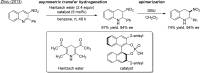
- Hara O.; Koshizawa T.; Makino K.; Kunimune I.; Namiki A.; Hamada Y. Tetrahedron 2007, 63, 6170–6181. 10.1016/j.tet.2007.03.078. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

