New Roads Leading to Old Destinations: Efflux Pumps as Targets to Reverse Multidrug Resistance in Bacteria
- PMID: 28294992
- PMCID: PMC6155429
- DOI: 10.3390/molecules22030468
New Roads Leading to Old Destinations: Efflux Pumps as Targets to Reverse Multidrug Resistance in Bacteria
Abstract
Multidrug resistance (MDR) has appeared in response to selective pressures resulting from the incorrect use of antibiotics and other antimicrobials. This inappropriate application and mismanagement of antibiotics have led to serious problems in the therapy of infectious diseases. Bacteria can develop resistance by various mechanisms and one of the most important factors resulting in MDR is efflux pump-mediated resistance. Because of the importance of the efflux-related multidrug resistance the development of new therapeutic approaches aiming to inhibit bacterial efflux pumps is a promising way to combat bacteria having over-expressed MDR efflux systems. The definition of an efflux pump inhibitor (EPI) includes the ability to render the bacterium increasingly more sensitive to a given antibiotic or even reverse the multidrug resistant phenotype. In the recent years numerous EPIs have been developed, although so far their clinical application has not yet been achieved due to their in vivo toxicity and side effects. In this review, we aim to give a short overview of efflux mediated resistance in bacteria, EPI compounds of plant and synthetic origin, and the possible methods to investigate and screen EPI compounds in bacterial systems.
Keywords: ABC-transporter; RND pump; efflux pump inhibitor (EPI); multidrug efflux pump; multidrug resistance; proton motive force.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
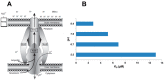
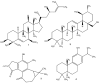
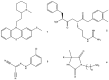
References
-
- World Health Organisation Antimicrobial resistance: Global Report on Surveillance. 2014. [(accessed on 13 March 2017)]. pp. 1–256. Available online: http://apps.who.int/iris/bitstream/10665/112642/1/9789241564748_eng.pdf?....
-
- World Economic Forum Global Risks. Eighth Edition. 2013. [(accessed on 13 March 2017)]. pp. 28–33. Available online: http://www3.weforum.org/docs/WEF_GlobalRisks_Report_2013.pdf.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

