Global Insight into Lysine Acetylation Events and Their Links to Biological Aspects in Beauveria bassiana, a Fungal Insect Pathogen
- PMID: 28295016
- PMCID: PMC5353618
- DOI: 10.1038/srep44360
Global Insight into Lysine Acetylation Events and Their Links to Biological Aspects in Beauveria bassiana, a Fungal Insect Pathogen
Abstract
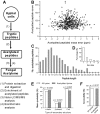
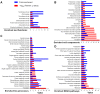
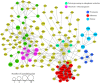
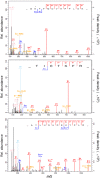
Lysine acetylation (Kac) events in filamentous fungi are poorly explored. Here we show a lysine acetylome generated by LC-MS/MS analysis of immunoaffinity-based Kac peptides from normal hyphal cells of Beauveria bassiana, a fungal entomopathogen. The acetylome comprised 283 Kac proteins and 464 Kac sites. These proteins were enriched to eight molecular functions, 20 cellular components, 27 biological processes, 20 KEGG pathways and 12 subcellular localizations. All Kac sites were characterized as six Kac motifs, including a novel motif (KacW) for 26 Kac sites of 17 unknown proteins. Many Kac sites were predicted to be multifunctional, largely expanding the fungal Kac events. Biological importance of identified Kac sites was confirmed through functional analysis of Kac sites on Pmt1 and Pmt4, two O-mannosyltransferases. Singular site mutations (K88R and K482R) of Pmt1 resulted in impaired conidiation, attenuated virulence and decreased tolerance to oxidation and cell wall perturbation. These defects were close to or more severe than those caused by the deletion of pmt1. The Pmt4 K360R mutation facilitated colony growth under normal and stressful conditions and enhanced the fungal virulence. Our findings provide the first insight into the Kac events of B. bassiana and their links to the fungal potential against insect pests.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Shahbazian M. D. & Grunstein M. Functions of site-specific histone acetylation and deacetylation. Annu Rev Biochem 76, 75–100 (2007). - PubMed
-
- Kim G. W. & Yang X. J. Comprehensive lysine acetylomes emerging from bacteria to humans. Trends Biochem Sci 36, 211–220 (2011). - PubMed
-
- Shimazu T., Hirschey M. D., Huang J. Y., Ho L. T. & Verdin E. Acetate metabolism and aging: An emerging connection. Mech Ageing Dev 131, 511–516 (2010). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

