Neuronal entry and high neurotoxicity of botulinum neurotoxin A require its N-terminal binding sub-domain
- PMID: 28295026
- PMCID: PMC5353748
- DOI: 10.1038/srep44474
Neuronal entry and high neurotoxicity of botulinum neurotoxin A require its N-terminal binding sub-domain
Abstract
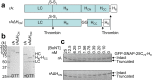
Botulinum neurotoxins (BoNTs) are the most toxic proteins known, due to inhibiting the neuronal release of acetylcholine and causing flaccid paralysis. Most BoNT serotypes target neurons by binding to synaptic vesicle proteins and gangliosides via a C-terminal binding sub-domain (HCC). However, the role of their conserved N-terminal sub-domain (HCN) has not been established. Herein, we created a mutant form of recombinant BoNT/A lacking HCN (rAΔHCN) and showed that the lethality of this mutant is reduced 3.3 × 104-fold compared to wild-type BoNT/A. Accordingly, low concentrations of rAΔHCN failed to bind either synaptic vesicle protein 2C or neurons, unlike the high-affinity neuronal binding obtained with 125I-BoNT/A (Kd = 0.46 nM). At a higher concentration, rAΔHCN did bind to cultured sensory neurons and cluster on the surface, even after 24 h exposure. In contrast, BoNT/A became internalised and its light chain appeared associated with the plasmalemma, and partially co-localised with vesicle-associated membrane protein 2 in some vesicular compartments. We further found that a point mutation (W985L) within HCN reduced the toxicity over 10-fold, while this mutant maintained the same level of binding to neurons as wild type BoNT/A, suggesting that HCN makes additional contributions to productive internalization/translocation steps beyond binding to neurons.
Conflict of interest statement
The authors declare that this research was supported in part by Allergan Inc. The funder had no role in study design, data collection and analysis or preparation of the manuscript.
Figures




Similar articles
-
Double receptor anchorage of botulinum neurotoxins accounts for their exquisite neurospecificity.Curr Top Microbiol Immunol. 2013;364:61-90. doi: 10.1007/978-3-642-33570-9_4. Curr Top Microbiol Immunol. 2013. PMID: 23239349
-
Botulinum neurotoxin serotype D attacks neurons via two carbohydrate-binding sites in a ganglioside-dependent manner.Biochem J. 2010 Oct 15;431(2):207-16. doi: 10.1042/BJ20101042. Biochem J. 2010. PMID: 20704566
-
Glycosylated SV2 and gangliosides as dual receptors for botulinum neurotoxin serotype F.Biochemistry. 2009 Jun 23;48(24):5631-41. doi: 10.1021/bi9002138. Biochemistry. 2009. PMID: 19476346 Free PMC article.
-
Association of botulinum neurotoxins with synaptic vesicle protein complexes.Toxicon. 2009 Oct;54(5):570-4. doi: 10.1016/j.toxicon.2009.01.040. Epub 2009 Apr 9. Toxicon. 2009. PMID: 19362106 Free PMC article. Review.
-
8-Hydroxyquinoline and hydroxamic acid inhibitors of botulinum neurotoxin BoNT/A.Curr Top Med Chem. 2014;14(18):2094-102. doi: 10.2174/1568026614666141022095114. Curr Top Med Chem. 2014. PMID: 25335884 Review.
Cited by
-
Activity of botulinum neurotoxin X and its structure when shielded by a non-toxic non-hemagglutinin protein.Commun Chem. 2024 Aug 13;7(1):179. doi: 10.1038/s42004-024-01262-8. Commun Chem. 2024. PMID: 39138288 Free PMC article.
-
Botulinum Neurotoxin Chimeras Suppress Stimulation by Capsaicin of Rat Trigeminal Sensory Neurons In Vivo and In Vitro.Toxins (Basel). 2022 Feb 4;14(2):116. doi: 10.3390/toxins14020116. Toxins (Basel). 2022. PMID: 35202143 Free PMC article.
-
The 25 kDa HCN Domain of Clostridial Neurotoxins Is Indispensable for Their Neurotoxicity.Toxins (Basel). 2020 Nov 26;12(12):743. doi: 10.3390/toxins12120743. Toxins (Basel). 2020. PMID: 33255952 Free PMC article.
-
Novel platform for engineering stable and effective vaccines against botulinum neurotoxins A, B and E.Front Immunol. 2024 Sep 9;15:1469919. doi: 10.3389/fimmu.2024.1469919. eCollection 2024. Front Immunol. 2024. PMID: 39315101 Free PMC article.
-
The structure of the tetanus toxin reveals pH-mediated domain dynamics.EMBO Rep. 2017 Aug;18(8):1306-1317. doi: 10.15252/embr.201744198. Epub 2017 Jun 23. EMBO Rep. 2017. PMID: 28645943 Free PMC article.
References
-
- Dolly J. O., Wang J., Zurawski T. H. & Meng J. Novel therapeutics based on recombinant botulinum neurotoxins to normalize the release of transmitters and pain mediators. FEBS J 278, 4454–66 (2011). - PubMed
-
- Dorizas A., Krueger N. & Sadick N. S. Aesthetic uses of the botulinum toxin. Dermatol Clin 32, 23–36 (2014). - PubMed
-
- Hexsel C., Hexsel D., Porto M. D., Schilling J. & Siega C. Botulinum toxin type A for aging face and aesthetic uses. Dermatol Ther 24, 54–61 (2011). - PubMed
-
- Gady J. & Ferneini E. M. Botulinum toxin A and headache treatment. Conn Med 77, 165–6 (2013). - PubMed
-
- Welch M. J., Purkiss J. R. & Foster K. A. Sensitivity of embryonic rat dorsal root ganglia neurons to Clostridium botulinum neurotoxins. Toxicon 38, 245–58 (2000). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

