TRAF3/CYLD mutations identify a distinct subset of human papillomavirus-associated head and neck squamous cell carcinoma
- PMID: 28295222
- PMCID: PMC5419871
- DOI: 10.1002/cncr.30570
TRAF3/CYLD mutations identify a distinct subset of human papillomavirus-associated head and neck squamous cell carcinoma
Abstract
Background: The incidence of human papillomavirus (HPV)-associated (HPV-positive) head and neck squamous cell carcinoma (HNSCC) of the oropharynx has dramatically increased over the last decade and continues to rise. Newly diagnosed HPV-positive HNSCCs in the United States currently outnumber any other HPV-associated cancers, including cervical cancer. Despite introduction of the HPV vaccine, the epidemic of HPV-positive HNSCC is expected to continue for approximately 60 years. Compared with patients who have tobacco-associated HNSCC, those who have HPV-positive HNSCC have better overall survival and response to treatment. Current treatment, including chemotherapy and radiation therapy, is associated with lifelong morbidity, and there are limited treatments and no curative options for patients who develop recurrent metastatic disease. Therapeutic de-escalation (decreased radiation dose) is being tested through clinical trials; however, those studies select patients based solely on tumor and patient smoking characteristics. Mechanisms of HPV-driven carcinogenesis in HNSCC are not well understood, which limits new therapeutic strategies and hinders the appropriate selection of patients for de-escalation therapy.
Methods: The authors analyzed HNSCC data from The Cancer Genome Atlas to identify molecular characteristics that correlate with outcomes and integration status of the HPV genome.
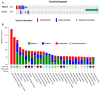
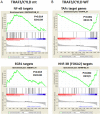
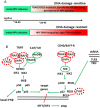
Results: The current investigations identified a subset of HPV-positive HNSCCs with mutations in the genes TRAF3 (tumor necrosis factor receptor-associated factor 3) and CYLD (cylindromatosis lysine 63 deubiquitinase). Defects in TRAF3 and CYLD correlated with the activation of transcriptional factor nuclear factor κB, episomal HPV status of tumors, and improved patient survival.
Conclusions: Defects in TRAF3/CYLD were accompanied with the activation of nuclear factor κB signaling and maintenance of episomal HPV in tumors, suggesting that these mutations may support an alternative mechanism of HPV tumorigenesis in head and neck tumors. Cancer 2017;123:1778-1790. © 2017 The Authors. Cancer published by Wiley Periodicals, Inc. on behalf of American Cancer Society. This is an open access article under the terms of the Creative Commons Attribution NonCommercial License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited and is not used for commercial purposes.
Keywords: head and neck squamous cell carcinoma; human papillomavirus (HPV); nuclear factor κB (NF-κB); prognosis; tumor necrosis factor receptor-associated factor 3 (TRAF3).
© 2017 The Authors. Cancer published by Wiley Periodicals, Inc. on behalf of American Cancer Society.
Figures



Comment in
-
Genetic alterations in TRAF3 and CYLD that regulate nuclear factor κB and interferon signaling define head and neck cancer subsets harboring human papillomavirus.Cancer. 2017 May 15;123(10):1695-1698. doi: 10.1002/cncr.30659. Epub 2017 Mar 13. Cancer. 2017. PMID: 28295216 Free PMC article.
Similar articles
-
NF-κB over-activation portends improved outcomes in HPV-associated head and neck cancer.Oncotarget. 2022 May 24;13:707-722. doi: 10.18632/oncotarget.28232. eCollection 2022. Oncotarget. 2022. PMID: 35634245 Free PMC article.
-
Noncanonical HPV carcinogenesis drives radiosensitization of head and neck tumors.Proc Natl Acad Sci U S A. 2023 Aug 8;120(32):e2216532120. doi: 10.1073/pnas.2216532120. Epub 2023 Jul 31. Proc Natl Acad Sci U S A. 2023. PMID: 37523561 Free PMC article.
-
Attenuated TRAF3 Fosters Activation of Alternative NF-κB and Reduced Expression of Antiviral Interferon, TP53, and RB to Promote HPV-Positive Head and Neck Cancers.Cancer Res. 2018 Aug 15;78(16):4613-4626. doi: 10.1158/0008-5472.CAN-17-0642. Epub 2018 Jun 19. Cancer Res. 2018. PMID: 29921694 Free PMC article.
-
The molecular mechanism of human papillomavirus-induced carcinogenesis in head and neck squamous cell carcinoma.Int J Clin Oncol. 2016 Oct;21(5):819-826. doi: 10.1007/s10147-016-1005-x. Epub 2016 Jun 23. Int J Clin Oncol. 2016. PMID: 27339270 Review.
-
NRF2, p53, and p16: Predictive biomarkers to stratify human papillomavirus associated head and neck cancer patients for de-escalation of cancer therapy.Crit Rev Oncol Hematol. 2020 Apr;148:102885. doi: 10.1016/j.critrevonc.2020.102885. Epub 2020 Feb 1. Crit Rev Oncol Hematol. 2020. PMID: 32062315 Review.
Cited by
-
Treating Head and Neck Cancer in the Age of Immunotherapy: A 2023 Update.Drugs. 2023 Feb;83(3):217-248. doi: 10.1007/s40265-023-01835-2. Epub 2023 Jan 16. Drugs. 2023. PMID: 36645621 Review.
-
Characterization of cancer genomic heterogeneity by next-generation sequencing advances precision medicine in cancer treatment.Precis Clin Med. 2018 Jun;1(1):29-48. doi: 10.1093/pcmedi/pby007. Epub 2018 Jun 14. Precis Clin Med. 2018. PMID: 30687561 Free PMC article. Review.
-
Genetically engineered mouse models of head and neck cancers.Oncogene. 2023 Aug;42(35):2593-2609. doi: 10.1038/s41388-023-02783-7. Epub 2023 Jul 20. Oncogene. 2023. PMID: 37474617 Free PMC article. Review.
-
The Pivotal Role of DNA Repair in Infection Mediated-Inflammation and Cancer.Front Microbiol. 2018 Apr 11;9:663. doi: 10.3389/fmicb.2018.00663. eCollection 2018. Front Microbiol. 2018. PMID: 29696001 Free PMC article. Review.
-
The Met1-linked ubiquitin machinery in inflammation and infection.Cell Death Differ. 2021 Feb;28(2):557-569. doi: 10.1038/s41418-020-00702-x. Epub 2021 Jan 20. Cell Death Differ. 2021. PMID: 33473179 Free PMC article. Review.
References
-
- Hunter KD, Parkinson EK, Harrison PR. Profiling early head and neck cancer. Nat Rev Cancer. 2005;5:127–135. - PubMed
-
- Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–289. - PubMed
-
- Sturgis EM, Cinciripini PM. Trends in head and neck cancer incidence in relation to smoking prevalence: an emerging epidemic of human papillomavirus‐associated cancers? Cancer. 2007;110:1429–1435. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

