Cav 1.3 channels play a crucial role in the formation of paroxysmal depolarization shifts in cultured hippocampal neurons
- PMID: 28295232
- PMCID: PMC7116787
- DOI: 10.1111/epi.13719
Cav 1.3 channels play a crucial role in the formation of paroxysmal depolarization shifts in cultured hippocampal neurons
Abstract
Objective: An increase of neuronal Cav 1.3 L-type calcium channels (LTCCs) has been observed in various animal models of epilepsy. However, LTCC inhibitors failed in clinical trials of epileptic treatment. There is compelling evidence that paroxysmal depolarization shifts (PDSs) involve Ca2+ influx through LTCCs. PDSs represent a hallmark of epileptiform activity. In recent years, a probable epileptogenic role for PDSs has been proposed. However, the implication of the two neuronal LTCC isoforms, Cav 1.2 and Cav 1.3, in PDSs remained unknown. Moreover, Ca2+ -dependent nonspecific cation (CAN) channels have also been suspected to contribute to PDSs. Nevertheless, direct experimental support of an important role of CAN channel activation in PDS formation is still lacking.
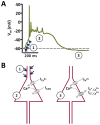
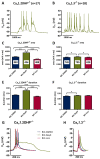
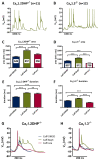
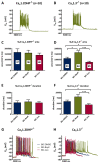
Methods: Primary neuronal networks derived from dissociated hippocampal neurons were generated from mice expressing a dihydropyridine-insensitive Cav 1.2 mutant (Cav 1.2DHP-/- mice) or from Cav 1.3-/- knockout mice. To investigate the role of Cav 1.2 and Cav 1.3, perforated patch-clamp recordings were made of epileptiform activity, which was elicited using either bicuculline or caffeine. LTCC activity was modulated using the dihydropyridines Bay K 8644 (agonist) and isradipine (antagonist).
Results: Distinct PDS could be elicited upon LTCC potentiation in Cav 1.2DHP-/- neurons but not in Cav 1.3-/- neurons. In contrast, when bicuculline led to long-lasting, seizure-like discharge events rather than PDS, these were prolonged in Cav 1.3-/- neurons but not in Cav 1.2DHP-/- neurons. Because only the Cav 1.2 isoform is functionally coupled to CAN channels in primary hippocampal networks, PDS formation does not require CAN channel activity.
Significance: Our data suggest that the LTCC requirement of PDS relates primarily to Cav 1.3 channels rather than to Cav 1.2 channels and CAN channels in hippocampal neurons. Hence, Cav 1.3 may represent a new therapeutic target for suppression of PDS development. The proposed epileptogenic role of PDSs may allow for a prophylactic rather than the unsuccessful seizure suppressing application of LTCC inhibitors.
Keywords: CAN channels; Epileptogenesis; L-type voltage-gated calcium channels; Primary cultured hippocampal neurons.
Wiley Periodicals, Inc. © 2017 International League Against Epilepsy.
Conflict of interest statement
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Figures

References
-
- Calin-Jageman I, Lee A. Cav1 L-type Ca2+ channel signaling complexes in neurons. J Neurochem. 2008;105:573–583. - PubMed
-
- Zhang Z, Séguéla P. Metabotropic induction of persistent activity in layers II/III of anterior cingulate cortex. Cereb Cortex. 2010;20:2948–2957. - PubMed
-
- Kulak W, Sobaniec W, Wojtal K, et al. Calcium modulation in epilepsy. Pol J Pharmacol. 2004;56:29–41. - PubMed
-
- Amano H, Amano T, Matsubayashi H, et al. Enhanced calcium influx in hippocampal CA3 neurons of spontaneously epileptic rats. Epilepsia. 2001;42:345–350. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

