Anatomy of the lobula complex in the brain of the praying mantis compared to the lobula complexes of the locust and cockroach
- PMID: 28295329
- PMCID: PMC5435961
- DOI: 10.1002/cne.24208
Anatomy of the lobula complex in the brain of the praying mantis compared to the lobula complexes of the locust and cockroach
Abstract
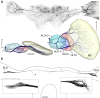
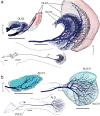
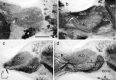
The praying mantis is an insect which relies on vision for capturing prey, avoiding being eaten and for spatial orientation. It is well known for its ability to use stereopsis for estimating the distance of objects. The neuronal substrate mediating visually driven behaviors, however, is not very well investigated. To provide a basis for future functional studies, we analyzed the anatomical organization of visual neuropils in the brain of the praying mantis Hierodula membranacea and provide supporting evidence from a second species, Rhombodera basalis, with particular focus on the lobula complex (LOX). Neuropils were three-dimensionally reconstructed from synapsin-immunostained whole mount brains. The neuropil organization and the pattern of γ-aminobutyric acid immunostaining of the medulla and LOX were compared between the praying mantis and two related polyneopteran species, the Madeira cockroach and the desert locust. The investigated visual neuropils of the praying mantis are highly structured. Unlike in most insects the LOX of the praying mantis consists of five nested neuropils with at least one neuropil not present in the cockroach or locust. Overall, the mantis LOX is more similar to the LOX of the locust than the more closely related cockroach suggesting that the sensory ecology plays a stronger role than the phylogenetic distance of the three species in structuring this center of visual information processing.
Keywords: RRID: AB_2313575; RRID: AB_2314457; RRID: AB_2315056; RRID: AB_2315425; RRID: AB_2336990; RRID: AB_2337244; RRID: AB_2338006; RRID: AB_2338713; RRID: AB_261363; RRID: nif-0000-00262; cockroach; insect visual system; lobula complex; locust; praying mantis.
© 2017 The Authors The Journal of Comparative Neurology Published by Wiley Periodicals, Inc.
Figures






References
-
- Beetz, M. J. , el Jundi, B. , Heinze, S. , & Homberg, U. (2015). Topographic organization and possible function of the posterior optic tubercles in the brain of the desert locust Schistocerca gregaria . The Journal of Comparative Neurology, 523, 1589–1607. - PubMed
-
- Berger, F. A. (1985). Morphologie und Physiologie einiger visueller Interneuronen in den optischen Ganglien der Gottesanbeterin Mantis religiosa. (Doctoral dissertation). University of Düsseldorf, Düsseldorf, Germany.
-
- Borst, A. , Haag, J. , & Reiff, D. F. (2010). Fly motion vision. Annual Review of Neuroscience, 33, 49–70. - PubMed
-
- Borst, A. , & Helmstaedter, M. (2014). Common circuit design in fly and mammalian motion vision. Nature Neuroscience, 18, 1067–1076. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

