Adipose tissue NAD+ biology in obesity and insulin resistance: From mechanism to therapy
- PMID: 28295415
- PMCID: PMC5469033
- DOI: 10.1002/bies.201600227
Adipose tissue NAD+ biology in obesity and insulin resistance: From mechanism to therapy
Abstract
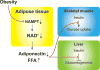
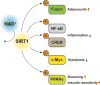
Nicotinamide adenine dinucleotide (NAD+ ) biosynthetic pathway, mediated by nicotinamide phosphoribosyltransferase (NAMPT), a key NAD+ biosynthetic enzyme, plays a pivotal role in controlling many biological processes, such as metabolism, circadian rhythm, inflammation, and aging. Over the past decade, NAMPT-mediated NAD+ biosynthesis, together with its key downstream mediator, namely the NAD+ -dependent protein deacetylase SIRT1, has been demonstrated to regulate glucose and lipid metabolism in a tissue-dependent manner. These discoveries have provided novel mechanistic and therapeutic insights into obesity and its metabolic complications, such as insulin resistance, an important risk factor for developing type 2 diabetes and cardiovascular disease. This review will focus on the importance of adipose tissue NAMPT-mediated NAD+ biosynthesis and SIRT1 in the pathophysiology of obesity and insulin resistance. We will also critically explore translational and clinical aspects of adipose tissue NAD+ biology.
Keywords: NAD+; NAMPT; PPARγ; SIRT1; adipose tissue; insulin resistance; obesity.
© 2017 WILEY Periodicals, Inc.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures

References
-
- Caballero B. The global epidemic of obesity: an overview. Epidemiol Rev. 2007;29:1–5. - PubMed
-
- Swinburn BA, Sacks G, Hall KD, McPherson K, et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378:804–14. - PubMed
-
- Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–9. quiz 20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

