Hepatic mitochondrial DNA/Toll-like receptor 9/MicroRNA-223 forms a negative feedback loop to limit neutrophil overactivation and acetaminophen hepatotoxicity in mice
- PMID: 28295449
- PMCID: PMC5481471
- DOI: 10.1002/hep.29153
Hepatic mitochondrial DNA/Toll-like receptor 9/MicroRNA-223 forms a negative feedback loop to limit neutrophil overactivation and acetaminophen hepatotoxicity in mice
Abstract
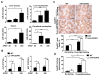
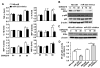
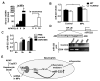
Acetaminophen (APAP) overdose is a leading cause of acute liver failure worldwide, in which mitochondrial DNA (mtDNA) released by damaged hepatocytes activates neutrophils through binding of Toll-like receptor 9 (TLR9), further aggravating liver injury. Here, we demonstrated that mtDNA/TLR9 also activates a negative feedback pathway through induction of microRNA-223 (miR-223) to limit neutrophil overactivation and liver injury. After injection of APAP in mice, levels of miR-223, the most abundant miRNAs in neutrophils, were highly elevated in neutrophils. Disruption of the miR-223 gene exacerbated APAP-induced hepatic neutrophil infiltration, oxidative stress, and injury and enhanced TLR9 ligand-mediated activation of proinflammatory mediators in neutrophils. An additional deletion of the intercellular adhesion molecule 1 (ICAM-1) gene ameliorated APAP-induced neutrophil infiltration and liver injury in miR-223 knockout mice. In vitro experiments revealed that miR-223-deficient neutrophils were more susceptible to TLR9 agonist-mediated induction of proinflammatory mediators and nuclear factor kappa B (NF-κB) signaling, whereas overexpression of miR-223 attenuated these effects in neutrophils. Moreover, inhibition of TLR9 signaling by either treatment with a TLR9 inhibitor or by disruption of TLR9 gene partially, but significantly, suppressed miR-223 expression in neutrophils post-APAP injection. In contrast, activation of TLR9 up-regulated miR-223 expression in neutrophils in vivo and in vitro. Mechanistically, activation of TLR9 up-regulated miR-223 by enhancing NF-κB binding on miR-223 promoter, whereas miR-223 attenuated TLR9/NF-κB-mediated inflammation by targeting IκB kinase α expression. Collectively, up-regulation of miR-223 plays a key role in terminating the acute neutrophilic response and is a therapeutic target for treatment of APAP-induced liver failure. (Hepatology 2017;66:220-234).
© 2017 by the American Association for the Study of Liver Diseases. This article has been contributed to by U.S. Government employees and their work is in the public domain in the USA.
Conflict of interest statement
Figures
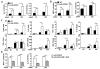
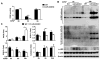
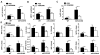

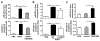
Similar articles
-
Chemokines and mitochondrial products activate neutrophils to amplify organ injury during mouse acute liver failure.Hepatology. 2012 Nov;56(5):1971-82. doi: 10.1002/hep.25801. Epub 2012 Aug 21. Hepatology. 2012. PMID: 22532075
-
Hepatic DNA deposition drives drug-induced liver injury and inflammation in mice.Hepatology. 2015 Jan;61(1):348-60. doi: 10.1002/hep.27216. Epub 2014 Jul 31. Hepatology. 2015. PMID: 24824608
-
Aryl Hydrocarbon Receptor Activity in Hepatocytes Sensitizes to Hyperacute Acetaminophen-Induced Hepatotoxicity in Mice.Cell Mol Gastroenterol Hepatol. 2021;11(2):371-388. doi: 10.1016/j.jcmgh.2020.09.002. Epub 2020 Sep 12. Cell Mol Gastroenterol Hepatol. 2021. PMID: 32932016 Free PMC article.
-
Gab1 adaptor protein acts as a gatekeeper to balance hepatocyte death and proliferation during acetaminophen-induced liver injury in mice.Hepatology. 2016 Apr;63(4):1340-55. doi: 10.1002/hep.28410. Epub 2016 Jan 22. Hepatology. 2016. PMID: 26680679
-
Free cholesterol accumulation in liver sinusoidal endothelial cells exacerbates acetaminophen hepatotoxicity via TLR9 signaling.J Hepatol. 2017 Oct;67(4):780-790. doi: 10.1016/j.jhep.2017.05.020. Epub 2017 May 26. J Hepatol. 2017. PMID: 28554874
Cited by
-
Role of Noncoding RNAs in Acetaminophen-Induced Liver Injury.Gene Expr. 2021 Jun 11;20(3):179-188. doi: 10.3727/105221621X16165282414118. Epub 2021 Mar 23. Gene Expr. 2021. PMID: 33757622 Free PMC article. Review.
-
Preliminary Study on Hepatoprotective Effect and Mechanism of (-)-Epigallocatechin-3-gallate against Acetaminophen-induced Liver Injury in Rats.Iran J Pharm Res. 2021 Summer;20(3):46-56. doi: 10.22037/ijpr.2020.112727.13918. Iran J Pharm Res. 2021. PMID: 34903968 Free PMC article.
-
Liver-specific deletion of mechanistic target of rapamycin does not protect against acetaminophen-induced liver injury in mice.Liver Res. 2021 Jun;5(2):79-87. doi: 10.1016/j.livres.2021.03.001. Epub 2021 Mar 19. Liver Res. 2021. PMID: 34504721 Free PMC article.
-
TLR9 in MAFLD and NASH: At the Intersection of Inflammation and Metabolism.Front Endocrinol (Lausanne). 2021 Jan 29;11:613639. doi: 10.3389/fendo.2020.613639. eCollection 2020. Front Endocrinol (Lausanne). 2021. PMID: 33584545 Free PMC article. Review.
-
Recent insights into the pathogeneses and therapeutic targets of liver diseases: Summary of the 4th Chinese American Liver Society/Society of Chinese Bioscientists in America Hepatology Division Symposium in 2021.Liver Res. 2022 Mar;6(1):50-57. doi: 10.1016/j.livres.2022.01.002. Epub 2022 Jan 29. Liver Res. 2022. PMID: 35747395 Free PMC article.
References
-
- Bunchorntavakul C, Reddy KR. Acetaminophen-related hepatotoxicity. Clin Liver Dis. 2013;17:587–607. viii. - PubMed
-
- Liu ZX, Kaplowitz N. Role of innate immunity in acetaminophen-induced hepatotoxicity. Expert Opin Drug Metab Toxicol. 2006;2:493–503. - PubMed
-
- Mossanen JC, Krenkel O, Ergen C, Govaere O, Liepelt A, Puengel T, et al. Chemokine (C-C motif) receptor 2-positive monocytes aggravate the early phase of acetaminophen-induced acute liver injury. Hepatology. 2016;64:1667–1682. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

