Immune phenotype and function of natural killer and T cells in chronic hepatitis C patients who received a single dose of anti-MicroRNA-122, RG-101
- PMID: 28295463
- PMCID: PMC5850982
- DOI: 10.1002/hep.29148
Immune phenotype and function of natural killer and T cells in chronic hepatitis C patients who received a single dose of anti-MicroRNA-122, RG-101
Abstract
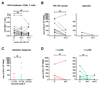
MicroRNA-122 is an important host factor for the hepatitis C virus (HCV). Treatment with RG-101, an N-acetylgalactosamine-conjugated anti-microRNA-122 oligonucleotide, resulted in a significant viral load reduction in patients with chronic HCV infection. Here, we analyzed the effects of RG-101 therapy on antiviral immunity. Thirty-two chronic HCV patients infected with HCV genotypes 1, 3, and 4 received a single subcutaneous administration of RG-101 at 2 mg/kg (n = 14) or 4 mg/kg (n = 14) or received a placebo (n = 2/dosing group). Plasma and peripheral blood mononuclear cells were collected at multiple time points, and comprehensive immunological analyses were performed. Following RG-101 administration, HCV RNA declined in all patients (mean decline at week 2, 3.27 log10 IU/mL). At week 8 HCV RNA was undetectable in 15/28 patients. Plasma interferon-γ-induced protein 10 (IP-10) levels declined significantly upon dosing with RG-101. Furthermore, the frequency of natural killer (NK) cells increased, the proportion of NK cells expressing activating receptors normalized, and NK cell interferon-γ production decreased after RG-101 dosing. Functional HCV-specific interferon-γ T-cell responses did not significantly change in patients who had undetectable HCV RNA levels by week 8 post-RG-101 injection. No increase in the magnitude of HCV-specific T-cell responses was observed at later time points, including 3 patients who were HCV RNA-negative 76 weeks postdosing.
Conclusion: Dosing with RG-101 is associated with a restoration of NK-cell proportions and a decrease of NK cells expressing activation receptors; however, the magnitude and functionality of ex vivo HCV-specific T-cell responses did not increase following RG-101 injection, suggesting that NK cells, but not HCV adaptive immunity, may contribute to HCV viral control following RG-101 therapy. (Hepatology 2017;66:57-68).
© 2017 The Authors. Hepatology published by Wiley Periodicals, Inc., on behalf of the American Association for the Study of Liver Diseases.
Conflict of interest statement
Figures




References
-
- Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61:S58–S68. - PubMed
-
- van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F, et al. Association Between Sustained Virological and Advanced Hepatic Fibrosis. J Am Med Assoc. 2012;308:2584–2593. - PubMed
-
- Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-ytter Y, et al. Eradication of Hepatitis C Virus Infection and the Development of Hepatocellular Carcinoma. Ann Intern Med. 2013;158:329–337. - PubMed
-
- Pawlotsky JM. New hepatitis C therapies: The toolbox, strategies, and challenges. Gastroenterology. 2014;146:1176–1192. - PubMed
-
- Lindenbach BD. What’s next for hepatitis C virus research? Hepatology. 2016;63:1408–1410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

