Chemical synthesis of long RNAs with terminal 5'-phosphate groups
- PMID: 28295757
- PMCID: PMC5413853
- DOI: 10.1002/chem.201700514
Chemical synthesis of long RNAs with terminal 5'-phosphate groups
Abstract
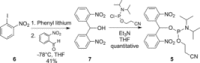
Long structured RNAs are useful biochemical and biological tools. They are usually prepared enzymatically, but this precludes their site-specific modification with functional groups for chemical biology studies. One solution is to perform solid-phase synthesis of multiple RNAs loaded with 5'-terminal phosphate groups, so that RNAs can be concatenated using template ligation reactions. However, there are currently no readily available reagents suitable for the incorporation of the phosphate group into long RNAs by solid-phase synthesis. Here we describe an easy-to-prepare phosphoramidite reagent suitable for the chemical introduction of 5'-terminal phosphate groups into long RNAs. The phosphate is protected by a dinitrobenzhydryl group that serves as an essential lipophilic group for the separation of oligonucleotide by-products. The phosphate is unmasked quantitatively by brief UV irradiation. We demonstrate the value of this reagent in the preparation of a library of long structured RNAs that are site-specifically modified with functional groups.
Keywords: RNA; dinitrobenzhydryl; phosphate; phosphoramidite; photolabile protecting group.
© 2017 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Figures

Similar articles
-
The synthesis of solid supports carrying base labile linkers to generate 3'-phosphate oligonucleotides.Bioorg Med Chem Lett. 2024 Sep 1;109:129819. doi: 10.1016/j.bmcl.2024.129819. Epub 2024 May 27. Bioorg Med Chem Lett. 2024. PMID: 38810710
-
Automated solid-phase synthesis of RNA oligonucleotides containing a nonbridging phosphorodithioate linkage via phosphorothioamidites.J Org Chem. 2012 Nov 2;77(21):9889-92. doi: 10.1021/jo301834p. Epub 2012 Oct 25. J Org Chem. 2012. PMID: 23050987 Free PMC article.
-
Efficient Automated Solid-Phase Synthesis of DNA and RNA 5'-Triphosphates.Chemistry. 2015 Nov 9;21(46):16421-6. doi: 10.1002/chem.201502844. Epub 2015 Aug 25. Chemistry. 2015. PMID: 26517040
-
Synthesis of Nucleobase-Modified RNA Oligonucleotides by Post-Synthetic Approach.Molecules. 2020 Jul 23;25(15):3344. doi: 10.3390/molecules25153344. Molecules. 2020. PMID: 32717917 Free PMC article. Review.
-
Chemical methods for the modification of RNA.Methods. 2019 May 15;161:64-82. doi: 10.1016/j.ymeth.2019.03.018. Epub 2019 Mar 21. Methods. 2019. PMID: 30905751 Review.
Cited by
-
Access to capped RNAs by chemical ligation.RSC Chem Biol. 2024 Sep 13;5(11):1104-10. doi: 10.1039/d4cb00165f. Online ahead of print. RSC Chem Biol. 2024. PMID: 39279877 Free PMC article.
-
Development of hydrophobic tag purifying monophosphorylated RNA for chemical synthesis of capped mRNA and enzymatic synthesis of circular mRNA.Nucleic Acids Res. 2024 Nov 11;52(20):12141-12157. doi: 10.1093/nar/gkae847. Nucleic Acids Res. 2024. PMID: 39414255 Free PMC article.
-
Site-Specific Fluorophore Labeling of Guanosines in RNA G-Quadruplexes.ACS Omega. 2019 May 14;4(5):8472-8479. doi: 10.1021/acsomega.9b00704. eCollection 2019 May 31. ACS Omega. 2019. PMID: 31459936 Free PMC article.
-
Enabling mRNA Therapeutics: Current Landscape and Challenges in Manufacturing.Biomolecules. 2023 Oct 9;13(10):1497. doi: 10.3390/biom13101497. Biomolecules. 2023. PMID: 37892179 Free PMC article. Review.
-
Thermostability, Tunability, and Tenacity of RNA as Rubbery Anionic Polymeric Materials in Nanotechnology and Nanomedicine-Specific Cancer Targeting with Undetectable Toxicity.Chem Rev. 2021 Jul 14;121(13):7398-7467. doi: 10.1021/acs.chemrev.1c00009. Epub 2021 May 26. Chem Rev. 2021. PMID: 34038115 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

