Recovery of shape and size in a developing organ pair
- PMID: 28295855
- PMCID: PMC5426968
- DOI: 10.1002/dvdy.24498
Recovery of shape and size in a developing organ pair
Abstract
Background: Paired organs in animals are largely bilaterally symmetric despite inherent noise in most biological processes. How is precise organ shape and size achieved during development despite this noise? Examining paired organ development is a challenge because it requires repeated quantification of two structures in parallel within living embryos. Here we combine bilateral quantification of morphology through time with asymmetric perturbations to study regulation of organ shape, size, and symmetry in developing organ pairs.
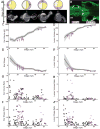
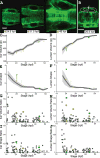
Results: We present quantitative live imaging tools to measure the shape and size of the developing inner ears on both the left and right side simultaneously over time. By quantifying variation between the left and right inner ear (intrinsic noise) and between different individuals (extrinsic noise), we find that initial variability decreases over time in normal development to achieve symmetry. Early asymmetry is increased by environmental stress, but symmetry is still recovered over subsequent developmental time. Using multiple unilateral perturbations including Fgf signaling and ultraviolet light, we find that growth can be adjusted to compensate for a range of initial size and shape differences.
Conclusions: We propose that symmetry in developmental systems does not emerge through precise deterministic bilateral development, but rather through feedback mechanisms that adjust morphogenesis rates to account for variation. Developmental Dynamics 246:451-465, 2016. © 2017 Wiley Periodicals, Inc.
Keywords: inner ear; otic vesicle; regeneration; regulation; size control; symmetry.
© 2017 Wiley Periodicals, Inc.
Figures

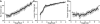





References
-
- Bryant PJ, Simpson P. Intrinsic and extrinsic control of growth in developing organs. The Quarterly Review of Biology. 1984;59:387–415. - PubMed
-
- Freeman DC, Brown ML, Dobson M, Jordan Y, Kizy A, Micallef C, Hancock LC, Graham JH, Emlen JM. Developmental instability: measures of resistance and resilience using pumpkin (Cucurbita pepo L.) Biological Journal of the Linnean Society. 2003;78:27–41.
-
- Freeman DC, Brown ML, Duda JJ, Graham JH, Emlen JM, Krzysik AJ, Balbach H, Kovavic DA, Zak JC. Leaf fluctuating asymmetry, soil disturbance and plant stress: a multiple year comparison using two herbs, Ipomoea pandurata and Cnidoscolus stimulosus. Ecological Indicators. 2005;5:85–95.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

