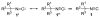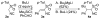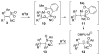Alkenyl Isocyanide Conjugate Additions: A Rapid Route to γ-Carbolines
- PMID: 28295938
- PMCID: PMC5667947
- DOI: 10.1002/anie.201612574
Alkenyl Isocyanide Conjugate Additions: A Rapid Route to γ-Carbolines
Abstract
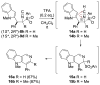
Isocyanides are exceptional building blocks, the wide deployment of which in multicomponent and metal-insertion reactions belies their limited availability. The first conjugate addition/alkylation to alkenyl isocyanides is described, which addresses this deficiency. An array of organolithiums, magnesiates, enolates, and metalated nitriles add conjugately to β- and β,β-disubstituted arylsulfonyl alkenyl isocyanides to rapidly assemble diverse isocyanide scaffolds. The intermediate metalated isocyanides are efficiently trapped with electrophiles to generate substituted isocyanides incorporating contiguous tri- and tetra-substituted centers. The substituted isocyanides are ideally functionalized for elaboration into synthetic targets as illustrated by the three-step synthesis of γ-carboline N-methyl ingenine B.
Keywords: conjugate addition; isocyanides; organometallics; synthetic methods; γ-carbolines.
© 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
References
-
- Suginome M, Ito Y. In: Science of Synthesis. Murahashi S-I, editor. Vol. 19. Thieme; Stuttgart: 2004. pp. 445–530.
-
- Ramozzi R, Chéron N, Braïda B, Hiberty PC, Fleurat-Lessard P. New J Chem. 2012;36:1137.
-
- Zhang B, Studer A. Chem Soc Rev. 2015;44:3505. - PubMed
-
- Zhu J, Wang Q, Wang M-X. Multicomponent Reactions in Organic Synthesis. VCH; Weinheim: 2014.
- Dömling A. Chem Rev. 2006;106:17. - PubMed
- Zhu J. Eur J Org Chem. 2003:1133.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources