Interactions of Renal-Clearable Gold Nanoparticles with Tumor Microenvironments: Vasculature and Acidity Effects
- PMID: 28295960
- PMCID: PMC5560109
- DOI: 10.1002/anie.201612647
Interactions of Renal-Clearable Gold Nanoparticles with Tumor Microenvironments: Vasculature and Acidity Effects
Abstract
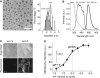
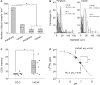
The success of nanomedicines in the clinic depends on our comprehensive understanding of nano-bio interactions in tumor microenvironments, which are characterized by dense leaky microvasculature and acidic extracellular pH (pHe ) values. Herein, we investigated the accumulation of ultrasmall renal-clearable gold NPs (AuNPs) with and without acidity targeting in xenograft mouse models of two prostate cancer types, PC-3 and LNCaP, with distinct microenvironments. Our results show that both sets of AuNPs could easily penetrate into the tumors but their uptake and retention were mainly dictated by the tumor microvasculature and the enhanced permeability and retention effect over the entire targeting process. On the other hand, increased tumor acidity indeed enhanced the uptake of AuNPs with acidity targeting, but only for a limited period of time. By making use of simple surface chemistry, these two effects can be synchronized in time for high tumor targeting, opening new possibilities to further improve the targeting efficiencies of nanomedicines.
Keywords: microvascular density; nanoparticles; renal clearance; tumor acidity; tumor targeting.
© 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, Chan WCW. Nat Rev Mater. 2016;1:16014.
-
- Kato Y, Ozawa S, Miyamoto C, Maehata Y, Suzuki A, Maeda T, Baba Y. Cancer Cell Int. 2013;13:89. - PMC - PubMed
- Danhier F, Feron O, Preat V. J Controlled Release. 2010;148:135–146. - PubMed
- Fukumura D, Jain RK. Microvasc Res. 2007;74:72–84. - PMC - PubMed
- Brown JM, Giaccia AJ. Cancer Res. 1998;58:1408–1416. - PubMed
-
- Yao L, Daniels J, Moshnikova A, Kuznetsov S, Ahmed A, Engelman DM, Reshetnyak YK, Andreev OA. Proc Natl Acad Sci USA. 2013;110:465–470. - PMC - PubMed
- Du JZ, Sun TM, Song WJ, Wu J, Wang J. Angew Chem Int Ed. 2010;49:3621–3626. - PubMed
- Angew Chem. 2010;122:3703–3708.
- Zhou KJ, Wang YG, Huang XN, Luby-Phelps K, Sumer BD, Gao JM. Angew Chem Int Ed. 2011;50:6109–6114. - PMC - PubMed
- Angew Chem. 2011;123:6233–6238.
- Crayton SH, Tsourkas A. ACS Nano. 2011;5:9592–9601. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

