Insulin-like growth factor 1 deficiency exacerbates hypertension-induced cerebral microhemorrhages in mice, mimicking the aging phenotype
- PMID: 28295976
- PMCID: PMC5418199
- DOI: 10.1111/acel.12583
Insulin-like growth factor 1 deficiency exacerbates hypertension-induced cerebral microhemorrhages in mice, mimicking the aging phenotype
Abstract
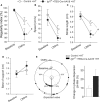
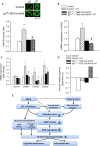
Clinical and experimental studies show that aging exacerbates hypertension-induced cerebral microhemorrhages (CMHs), which progressively impair neuronal function. There is growing evidence that aging promotes insulin-like growth factor 1 (IGF-1) deficiency, which compromises multiple aspects of cerebromicrovascular and brain health. To determine the role of IGF-1 deficiency in the pathogenesis of CMHs, we induced hypertension in mice with liver-specific knockdown of IGF-1 (Igf1f/f + TBG-Cre-AAV8) and control mice by angiotensin II plus l-NAME treatment. In IGF-1-deficient mice, the same level of hypertension led to significantly earlier onset and increased incidence and neurological consequences of CMHs, as compared to control mice, as shown by neurological examination, gait analysis, and histological assessment of CMHs in serial brain sections. Previous studies showed that in aging, increased oxidative stress-mediated matrix metalloprotease (MMP) activation importantly contributes to the pathogenesis of CMHs. Thus, it is significant that hypertension-induced cerebrovascular oxidative stress and MMP activation were increased in IGF-1-deficient mice. We found that IGF-1 deficiency impaired hypertension-induced adaptive media hypertrophy and extracellular matrix remodeling, which together with the increased MMP activation likely also contributes to increased fragility of intracerebral arterioles. Collectively, IGF-1 deficiency promotes the pathogenesis of CMHs, mimicking the aging phenotype, which likely contribute to its deleterious effect on cognitive function. Therapeutic strategies that upregulate IGF-1 signaling in the cerebral vessels and/or reduce microvascular oxidative stress, and MMP activation may be useful for the prevention of CMHs, protecting cognitive function in high-risk elderly patients.
Keywords: arteriole; dementia; gait dysfunction; microbleed; oxidative stress.
© 2017 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Figures


References
-
- Bailey‐Downs LC, Mitschelen M, Sosnowska D, Toth P, Pinto JT, Ballabh P, Valcarcel‐Ares MN, Farley J, Koller A, Henthorn JC, Bass C, Sonntag WE, Ungvari Z, Csiszar A (2012) Liver‐specific knockdown of IGF‐1 decreases vascular oxidative stress resistance by impairing the Nrf2‐dependent antioxidant response: a novel model of vascular aging. J. Gerontol. Biol. Med. Sci. 67, 313–329. - PMC - PubMed
-
- Baumbach GL, Heistad DD (1989) Remodeling of cerebral arterioles in chronic hypertension. Hypertension 13, 968–972. - PubMed
-
- Choi P, Ren M, Phan TG, Callisaya M, Ly JV, Beare R, Chong W, Srikanth V (2012) Silent infarcts and cerebral microbleeds modify the associations of white matter lesions with gait and postural stability: population‐based study. Stroke 43, 1505–1510. - PubMed
-
- Csiszar A, Labinskyy N, Perez V, Recchia FA, Podlutsky A, Mukhopadhyay P, Losonczy G, Pacher P, Austad SN, Bartke A, Ungvari Z (2008) Endothelial function and vascular oxidative stress in long‐lived GH/IGF‐deficient Ames dwarf mice. Am. J. Physiol. Heart Circ. Physiol. 295, H1882–H1894. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

