Synthesis and Opioid Activity of Tyr1 -ψ[(Z)CF=CH]-Gly2 and Tyr1 -ψ[(S)/(R)-CF3 CH-NH]-Gly2 Leu-enkephalin Fluorinated Peptidomimetics
- PMID: 28296145
- PMCID: PMC5486982
- DOI: 10.1002/cmdc.201700103
Synthesis and Opioid Activity of Tyr1 -ψ[(Z)CF=CH]-Gly2 and Tyr1 -ψ[(S)/(R)-CF3 CH-NH]-Gly2 Leu-enkephalin Fluorinated Peptidomimetics
Abstract
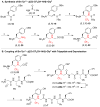
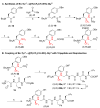
We describe the design, synthesis, and opioid activity of fluoroalkene (Tyr1 -ψ[(Z)CF=CH]-Gly2 ) and trifluoroethylamine (Tyr1 -ψ[(S)/(R)-CF3 CH-NH]-Gly2 ) analogues of the endogenous opioid neuropeptide, Leu-enkephalin. The fluoroalkene peptidomimetic exhibited low nanomolar functional activity (5.0±2 nm and 60±15 nm for δ- and μ-opioid receptors, respectively) with a μ/δ-selectivity ratio that mimics that of the natural peptide. However, the trifluoroethylamine peptidomimetics, irrespective of stereochemistry, did not activate the opioid receptors, which suggest that bulky CF3 substituents are not tolerated at this position.
Keywords: amide bonds; enkephalin; fluorine; opioids; peptidomimetics.
© 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures



Similar articles
-
Synthesis of Gly-ψ[(Z)CF═CH]-Phe, a Fluoroalkene Dipeptide Isostere, and Its Incorporation into a Leu-enkephalin Peptidomimetic.ACS Chem Neurosci. 2017 Jan 18;8(1):40-49. doi: 10.1021/acschemneuro.6b00163. Epub 2016 Nov 3. ACS Chem Neurosci. 2017. PMID: 27762555
-
Tyr1-ψ[( Z)CF═CH]-Gly2 Fluorinated Peptidomimetic Improves Distribution and Metabolism Properties of Leu-Enkephalin.ACS Chem Neurosci. 2018 Jul 18;9(7):1735-1742. doi: 10.1021/acschemneuro.8b00085. Epub 2018 Apr 19. ACS Chem Neurosci. 2018. PMID: 29648788 Free PMC article.
-
Opioid activity of 4-imidazolidinone positional analogues of Leu-Enkephalin.Bioorg Med Chem Lett. 2002 Nov 4;12(21):3175-8. doi: 10.1016/s0960-894x(02)00678-9. Bioorg Med Chem Lett. 2002. PMID: 12372527
-
In vitro agonist effects of nociceptin and [Phe(1)psi(CH(2)-NH)Gly(2)]nociceptin(1-13)NH(2) in the mouse and rat colon and the mouse vas deferens.Eur J Pharmacol. 1999 Dec 3;385(2-3):217-23. doi: 10.1016/s0014-2999(99)00700-1. Eur J Pharmacol. 1999. PMID: 10607879
-
The TIPP opioid peptide family: development of delta antagonists, delta agonists, and mixed mu agonist/delta antagonists.Biopolymers. 1999;51(6):411-25. doi: 10.1002/(SICI)1097-0282(1999)51:6<411::AID-BIP4>3.0.CO;2-Z. Biopolymers. 1999. PMID: 10797230 Review.
Cited by
-
Recent progress in the racemic and enantioselective synthesis of monofluoroalkene-based dipeptide isosteres.Beilstein J Org Chem. 2017 Dec 12;13:2637-2658. doi: 10.3762/bjoc.13.262. eCollection 2017. Beilstein J Org Chem. 2017. PMID: 29564002 Free PMC article. Review.
-
Searching for Synthetic Opioid Rescue Agents: Identification of a Potent Opioid Agonist with Reduced Respiratory Depression.J Med Chem. 2024 Jun 13;67(11):9173-9193. doi: 10.1021/acs.jmedchem.4c00333. Epub 2024 May 29. J Med Chem. 2024. PMID: 38810170 Free PMC article.
-
Synthesis of Leu-Enkephalin Peptidomimetics Containing Trifluoromethylalkenes as Amide Isopolar Mimics.J Fluor Chem. 2019 Feb;218:90-98. doi: 10.1016/j.jfluchem.2018.12.005. Epub 2018 Dec 11. J Fluor Chem. 2019. PMID: 31061541 Free PMC article.
-
Searching for Synthetic Opioid Rescue Agents. 2: Identification of an Ultra-Potent Synthetic Opioid Rescue Agent.J Med Chem. 2025 Jun 26;68(12):13057-13074. doi: 10.1021/acs.jmedchem.5c01108. Epub 2025 Jun 11. J Med Chem. 2025. PMID: 40499097
-
Modulating β-arrestin 2 recruitment at the δ- and μ-opioid receptors using peptidomimetic ligands.RSC Med Chem. 2021 Aug 16;12(11):1958-1967. doi: 10.1039/d1md00025j. eCollection 2021 Nov 17. RSC Med Chem. 2021. PMID: 34825191 Free PMC article.
References
-
- Gutstein H, Akil H. Opioid analgesics. New York: McGraw-Hill; 2001.
-
- Volkow ND, McLellan AT. N Engl J Med. 2015;374(13):1253–1263. - PubMed
-
- National Institute on Drug Abuse. National Institute on Drug Abuse; 2015. The latest prescription trends for controlled prescription drugs. ( http://www.drugabuse.gov/news-events/meetings-events/2015/09/latest-pres...)
-
- Bodnar RJ. Peptides. 2014;75:18–70. - PubMed
-
- H H, Warner M, Chen LH. NCHS Health E-Stat. Hyattsville, MD: National Centers for Health Statistics; 2014. Trends in drug-poisoning deaths involving opioid analgesics and heroin: United States, 1999–2012.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

