Conformational Populations of β-(1→4) O-Glycosidic Linkages Using Redundant NMR J-Couplings and Circular Statistics
- PMID: 28296420
- PMCID: PMC5553453
- DOI: 10.1021/acs.jpcb.7b02252
Conformational Populations of β-(1→4) O-Glycosidic Linkages Using Redundant NMR J-Couplings and Circular Statistics
Abstract
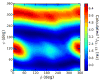
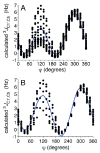
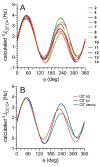
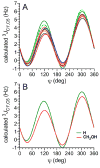
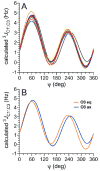
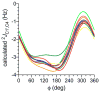
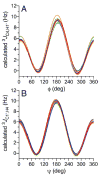
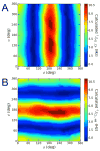
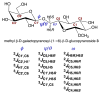
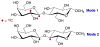
Twelve disaccharides containing β-(1→4) linkages and displaying systematic structural variations in the vicinity of these linkages were selectively labeled with 13C to facilitate measurements of multiple NMR spin-spin (scalar; J) coupling constants (JCH and JCC values) across their O-glycosidic linkages. Ensembles of spin-couplings (2JCOC, 3JCOCH, 3JCOCC) sensitive to the two linkage torsion angles, phi (ϕ) and psi (ψ), were analyzed by using parametrized equations obtained from density functional theory (DFT) calculations, Fredholm theory, and circular statistics to calculate experiment-based rotamer populations for ϕ and ψ in each disaccharide. With the statistical program MA'AT, torsion angles ϕ and ψ were modeled as a single von Mises distribution, which yielded two parameters, the mean position and the circular standard deviation (CSD) for each angle. The NMR-derived rotamer populations were compared to those obtained from 1 μs aqueous molecular dynamics (MD) simulations and crystallographic database statistical analyses. Conformer populations obtained exclusively from the MA'AT treatment of redundant J-couplings were in very good agreement with those obtained from the MD simulations, providing evidence that conformational populations can be determined by NMR for mobile molecular elements such as O-glycosidic linkages with minimal input from theory. The approach also provides an experimental means to validate the conformational preferences predicted from MD simulations. The conformational behaviors of ϕ in the 12 disaccharides were very similar, but those of ψ varied significantly, allowing a classification of the 12 disaccharides based on preferred linkage conformation in solution.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




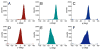




References
-
- Carver JP. Oligosaccharides: How Can Flexible Molecules Act as Signals? Pure Appl Chem. 1993;65:763–770.
-
- Homans SW. Conformation and Dynamics of Oligosaccharides in Solution. Glycobiology. 1993;3:551–555. - PubMed
-
- Yamamoto S, Zhang Y, Yamaguchi T, Kameda T, Kato K. Lanthanide-assisted NMR Evaluation of a Dynamic Ensemble of Oligosaccharide Conformations. Chem Commun. 2012;48:4752–4754. - PubMed
-
- Warner JB, Thalhauser C, Tao K, Sahagian GG. Role of N-Linked Oligosaccharide Flexibility in Mannose Phosphorylation of Lysosomal Enzyme Cathepsin L. J Biol Chem. 2002;277:41897–41905. - PubMed
-
- Woods RJ, Pathiaseril A, Wormald MR, Edge CJ, Dwek RA. The High Degree of Internal Flexibility Observed for an Oligomannose Oligosaccharide Does Not Alter the Overall Topology of the Molecule. Eur J Biochem. 1998;258:372–386. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

