Drifts in ADCC-related quality attributes of Herceptin®: Impact on development of a trastuzumab biosimilar
- PMID: 28296619
- PMCID: PMC5419076
- DOI: 10.1080/19420862.2017.1305530
Drifts in ADCC-related quality attributes of Herceptin®: Impact on development of a trastuzumab biosimilar
Abstract
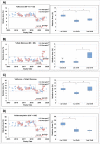
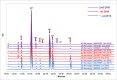
A biosimilar product needs to demonstrate biosimilarity to the originator reference product, and the quality profile of the latter should be monitored throughout the period of the biosimilar's development to match the quality attributes of the 2 products that relate to efficacy and safety. For the development of a biosimilar version of trastuzumab, the reference product, Herceptin®, was extensively characterized for the main physicochemical and biologic properties by standard or state-of-the-art analytical methods, using multiple lots expiring between March 2015 and December 2019. For lots with expiry dates up to July 2018, a high degree of consistency was observed for all the tested properties. However, among the lots expiring in August 2018 or later, a downward drift was observed in %afucose (G0+G1+G2). Furthermore, the upward drift of %high mannose (M5+M6) was observed in the lots with expiry dates from June 2019 to December 2019. As a result, the combination of %afucose and %high mannose showed 2 marked drifts in the lots with expiry dates from August 2018 to December 2019, which was supported by the similar trend of biologic data, such as FcγRIIIa binding and antibody-dependent cell-mediated cytotoxicity (ADCC) activity. Considering that ADCC is one of the clinically relevant mechanisms of action for trastuzumab, the levels of %afucose and %high mannose should be tightly monitored as critical quality attributes for biosimilar development of trastuzumab.
Keywords: ADCC; FcγRIIIa; Herceptin®; N-glycan; biosimilar; quality drift; trastuzumab.
Figures



References
-
- Committee for Medicinal Products for Human Use (CHMP) Guideline on similar biological medicinal products containing monoclonal antibodies-non clinical and clinical issues. EMEA/CHMP/BMWP/42832/2005 Rev1, European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP) 2014. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin...
-
- European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP) Guideline on similar biological medicinal product. CHMP/437/04 Rev. 1. European Medicines Agency 2005. Available from:http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin...
-
- Food and Drug Administration Center for Drug Evaluation and Research (FDA) Guidance for Industry: scientific considerations in demonstrating biosimilarity to a reference product. FDA-2011-D-0605, U.S. Department of Health and Human Services 2015. Available from: http://www.fda.gov/downloads/DrugsGuidanceComplianceRegulatoryInformatio...
-
- European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP) Guideline on similar biologic medicinal products containing biotechnology-derived protein as active substance: quality issue (revision 1). EMA/CHMP/BWP/247713/2012, European Medicines Agency 2014. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin...
-
- Ramanan S, Grampp G. Drift, evolution, and divergence in biologics and biosimilars manufacturing. BioDrugs 2014; 28(4):363-72; PMID:24567263; http://dx.doi.org/10.1007/s40259-014-0088-z - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
