DUSP11 - An RNA phosphatase that regulates host and viral non-coding RNAs in mammalian cells
- PMID: 28296624
- PMCID: PMC5785229
- DOI: 10.1080/15476286.2017.1306169
DUSP11 - An RNA phosphatase that regulates host and viral non-coding RNAs in mammalian cells
Abstract
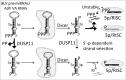
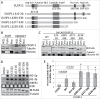
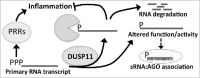
Dual-specificity phosphatase 11 (DUSP11) is a conserved protein tyrosine phosphatase (PTP) in metazoans. The cellular substrates and physiologic activities of DUSP11 remain largely unknown. In nematodes, DUSP11 is required for normal development and RNA interference against endogenous RNAs (endo-RNAi) via molecular mechanisms that are not well understood. However, mammals lack analogous endo-RNAi pathways and consequently, a role for DUSP11 in mammalian RNA silencing was unanticipated. Recent work from our laboratory demonstrated that DUSP11 activity alters the silencing potential of noncanonical viral miRNAs in mammalian cells. Our studies further uncovered direct cellular substrates of DUSP11 and suggest that DUSP11 is part of regulatory pathway that controls the abundance of select triphosphorylated noncoding RNAs. Here, we highlight recent findings and present new data that advance understanding of mammalian DUSP11 during gene silencing and discuss the emerging biological activities of DUSP11 in mammalian cells.
Keywords: Argonaute; BLV; DUSP11; PIR-1; RNAi; VA RNA; dicer; miRNA; miRNA biogenesis; triphosphate.
Figures

References
-
- Szymanski M, Barciszewska MZ, Erdmann VA, Barciszewski J. 5S ribosomal RNA database. Nucleic Acids Res 2002; 30(1):176-78; PMID:11752286; https://doi.org/10.1093/nar/30.1.176 - DOI - PMC - PubMed
-
- Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004; 116(2):281-97; PMID:14744438; https://doi.org/10.1016/S0092-8674(04)00045-5 - DOI - PubMed
-
- Kim VN. MicroRNA biogenesis: Coordinated cropping and dicing. Nat Rev Mol Cell Biol 2005; 6(5):376-85; PMID:15852042; https://doi.org/10.1038/nrm1644 - DOI - PubMed
-
- Julkunen I, Sareneva T, Pirhonen J, Ronni T, Melen K, Matikainen S. Molecular pathogenesis of influenza a virus infection and virus-induce regulation of cytokine gene expression. Cytokine Growth Factor Rev 2001; 12(2-3):171-80; PMID:11325600; https://doi.org/10.1016/S1359-6101(00)00026-5 - DOI - PubMed
-
- Hornung V, Ellegast J, Kim S, Brzozka K, Jung A, Kato H, Poeck H, Akira S, Conzelmann KK, Martin S, et al.. Triphosphate RNA is the ligand for RIG-I. Science 2006; 314(5801):994-7; PMID:17038590; https://doi.org/10.1126/science.1132505 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
