Effect on mother and child of eculizumab given before caesarean section in a patient with severe antiphospholipid syndrome: A case report
- PMID: 28296762
- PMCID: PMC5369917
- DOI: 10.1097/MD.0000000000006338
Effect on mother and child of eculizumab given before caesarean section in a patient with severe antiphospholipid syndrome: A case report
Abstract
Rationale: Antiphospholipid syndrome (APS) in pregnancy may trigger the life-threatening catastrophic antiphospholipid syndrome (CAPS). Complement activation is implicated in the pathogenesis, and inhibition of complement factor C5 is suggested as an additional treatment option.
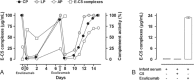
Patient concerns, diagnosis and interventions: We present a pregnant patient treated with the C5-inhibitor eculizumab due to high risk of developing devastating APS-related complications. The complement inhibitory effects of the treatment were examined both in the patient and the premature infant.
Outcomes: Complement activity in the mother recovered considerably faster than anticipated; however, no new thrombosis or CAPS developed during the last week of pregnancy or postpartum. Blood sampling from the umbilical vein and artery, and from the infant after delivery showed low complement activity; however, only 0.3% of the eculizumab concentration detected in the mother, consistent with low placental passage of eculizumab.
Lessons: The data underscore the importance of close monitoring of complement inhibition and individualizing dosage regimens in pregnant patients receiving eculizumab. We document how traditional functional complement activity tests cannot assess the effect of eculizumab in premature infants due to the very low levels of complement factors detected in this infant born in gestational week 33. Only trace amounts of eculizumab passed the placenta. In conclusion, complement C5 inhibition might be a safe candidate treatment option for APS during pregnancy and delivery, and additionally, enables prolongation of pregnancy with important weeks.
Conflict of interest statement
The authors have no funding and conflicts of interest to disclose.
Figures
References
-
- Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006;4:295–306. - PubMed
-
- Romay-Penabad Z, Carrera Marin AL, Willis R, et al. Complement C5-inhibitor rEV576 (coversin) ameliorates in-vivo effects of antiphospholipid antibodies. Lupus 2014;23:1324–6. - PubMed
-
- Giannakopoulos B, Krilis SA. The pathogenesis of the antiphospholipid syndrome. N Engl J Med 2013;368:1033–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

