Evaluating the immunogenicity and safety of a BiondVax-developed universal influenza vaccine (Multimeric-001) either as a standalone vaccine or as a primer to H5N1 influenza vaccine: Phase IIb study protocol
- PMID: 28296763
- PMCID: PMC5369918
- DOI: 10.1097/MD.0000000000006339
Evaluating the immunogenicity and safety of a BiondVax-developed universal influenza vaccine (Multimeric-001) either as a standalone vaccine or as a primer to H5N1 influenza vaccine: Phase IIb study protocol
Abstract
Introduction: Influenza is a major respiratory viral infection of humans with high mortality and morbidity rates and profound economic impact. Although influenza vaccines are generally updated yearly to match the viruses expected in the coming season, genetic mutation and reassortment can result in unexpected novel strains. Therefore, it is important to develop universal vaccines inducing protective immunity to such strains before they appear. This clinical trial is designed to evaluate the safety and immunogenicity of Multimeric-001 (M-001), which contains conserved epitopes of influenza A and B. M-001 is able to induce both humoral and cellular immunity and provides broad strain coverage.
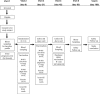
Methods: In a multicenter, randomized, double-blind, and controlled phase IIb trial, 222 healthy volunteers aged 18 to 60 years will be randomized into 3 groups (1:1:1) to receive either 2 intramuscular injections of 0.5 mg M-001 (arm 1), 1.0 mg M-001 (arm 2), or saline (arm 3-placebo), before receiving an investigational (whole virus, inactivated, aluminum phosphate gel [AlPO4]-adjuvanted) prepandemic influenza vaccine (H5N1). Primary outcomes are safety and cellular immune responses (cell-mediated immunity [CMI]) induced by M-001, evaluated by multiparametric flow cytometry of intracellular cytokines. The secondary outcome is the serum hemagglutination inhibition (HAI) titer toward the H5N1 vaccine strain. Additionally, exploratory outcomes include evaluation of CMI by quantitative reverse transcription polymerase chain reaction of cytokine mRNA, HAI titers toward H5-drifted strains, serum single radial hemolysis titers toward the H5N1 study vaccine, and the association between CMI markers and antibody response.
Discussion: There is a need for influenza vaccines that give the population a broader protection against multiple strains of influenza virus. M-001 might be such vaccine which will be tested in this current trial as a standalone vaccine and as a pandemic primer. Both cellular and humoral immune responses will be evaluated.
Trial registration: EudraCT number: 2015-001979-46.
Conflict of interest statement
TB-Y and SH are employees of BiondVax Pharmaceuticals Ltd. The other authors declare that they have no competing interests.
Figures
References
-
- Cox RJ, Brokstad KA, Ogra P. Influenza virus: immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scand J Immunol 2004;59:1–5. - PubMed
-
- World Health Organization. Influenza Virus Infections in Humans (February 2014). http://www.who.int/influenza/human_animal_interface/virology_laboratorie.... Accessed April 13, 2015.
-
- Rothberg MB, Haessler SD. Complications of seasonal and pandemic influenza. Crit Care Med 2010;38(suppl):e91–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical