Evaluation of intrathecal drug delivery system for intractable pain in advanced malignancies: A prospective cohort study
- PMID: 28296770
- PMCID: PMC5369925
- DOI: 10.1097/MD.0000000000006354
Evaluation of intrathecal drug delivery system for intractable pain in advanced malignancies: A prospective cohort study
Abstract
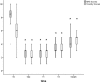
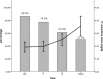
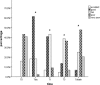
Pain is prevalent in advanced malignancies; however, some patients cannot get adequate pain relief by conservative routes of analgesic administration or experience serious side effects related to high dose of opioids. For those who have exhausted multimodal conservative analgesic, intrathecal drug delivery is an alternative intervention for truly effective pain management. The objective of this study was to evaluate the clinical efficacy and safety of intrathecal drug delivery system (IDDS) for the treatment of intractable pain in advanced cancer patients.A prospective cohort study was performed between July 2015 and October 2016. Fifty-three patients undergoing intractable cancer-related pain or intolerable drug-related adverse effects were recruited and received IDDS therapy with a patient-controlled intrathecal analgesia pump. The assessment was conducted during admission, in titration period, and followed up monthly to death by scheduled refill visits. Pain numeric rating scale scores, comprehensive toxicity scores, quality of life scores, systemic opioid use (basal and breakthrough dose), intrathecal morphine use (basal and patient-controlled intrathecal analgesia dose), and complications were recorded to evaluate the curative effect and safety.Between baseline and all subsequent follow-ups, statistically significant decreases in pain numeric rating scale scores and comprehensive toxicity scores were verified. A statistical improvement in quality of life scores was found after starting IDDS therapy. Both basal and breakthrough doses of systemic opioid showed a significant decrease during the follow-up period. And there was a modest escalation in the intrathecal morphine dose throughout the duration of study. No infective, device-related, and catheter-related complications were observed.The findings showed that IDDS therapy allowed for rapid and highly effective pain relief with less toxicity in comparison to conservative medications. Patients with advanced malignancies would also benefit from an improvement in the life quality after the procedure. IDDS therapy represented a valuable option for intractable cancer-related pain management.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
References
-
- van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, et al. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol 2007;18:1437–49. - PubMed
-
- Jadad AR, Browman GP. The WHO analgesic ladder for cancer pain management. Stepping up the quality of its evaluation. JAMA 1995;274:1870–3. - PubMed
-
- Walker VA, Hoskin PJ, Hanks GW, et al. Evaluation of WHO analgesic guidelines for cancer pain in a hospital-based palliative care unit. J Pain Symptom Manage 1988;3:145–9. - PubMed
-
- Zech DF, Grond S, Lynch J, et al. Validation of World Health Organization guidelines for cancer pain relief: a 10-year prospective study. Pain 1995;63:65–76. - PubMed
-
- Chen L, Sein M, Vo T, et al. Clinical interpretation of opioid tolerance versus opioid-induced hyperalgesia. J Opioid Manag 2014;10:383–93. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

