All-cause mortality in HIV-positive adults starting combination antiretroviral therapy: correcting for loss to follow-up
- PMID: 28296798
- PMCID: PMC5540664
- DOI: 10.1097/QAD.0000000000001321
All-cause mortality in HIV-positive adults starting combination antiretroviral therapy: correcting for loss to follow-up
Abstract
Objective: To estimate mortality in HIV-positive patients starting combination antiretroviral therapy (ART) and to discuss different approaches to calculating correction factors to account for loss to follow-up.
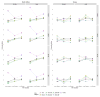
Methods: A total of 222 096 adult HIV-positive patients who started ART 2009-2014 in clinics participating in the International epidemiology Databases to Evaluate AIDS collaboration in 43 countries in sub-Saharan Africa, Asia Pacific, Latin America, and North America were included. To allow for underascertainment of deaths due to loss to follow-up, two correction factors (one for the period 0-6 months on ART and one for later periods) or 168 correction factors (combinations of two sexes, three time periods after ART initiation, four age groups, and seven CD4 groups) based on tracing patients lost in Kenya and data linkages in South Africa were applied. Corrected mortality rates were compared with a worst case scenario assuming all patients lost to follow-up had died.
Results: Loss to follow-up differed between regions; rates were lowest in central Africa and highest in east Africa. Compared with using two correction factors (1.64 for the initial ART period and 2.19 for later), applying 168 correction factors (range 1.03-4.75) more often resulted in implausible mortality rates that exceeded the worst case scenario. Corrected mortality rates varied widely, ranging from 0.2 per 100 person-years to 54 per 100 person-years depending on region and covariates.
Conclusion: Implausible rates were less common with the simpler approach based on two correction factors. The corrected mortality rates will be useful to international agencies, national programmes, and modellers.
Conflict of interest statement
ML received unrestricted grants from Boehringer Ingelhiem, Gilead Sciences, Merck Sharp & Dohme, Bristol-Myers Squibb, Janssen-Cilag, ViiV HealthCare, consultancy and presentation fees from Gilead Sciences, DSMB sitting fees from Sirtex Pty Ltd. All other authors have no conflict of interest to disclose.
Figures

References
-
- Egger M, May M, Chene G, Phillips AN, Ledergerber B, Dabis F, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–129. - PubMed
-
- Wilkinson LS, Skordis-Worrall J, Ajose O, Ford N, LSW, JS-W, et al. Self-transfer and mortality amongst adults lost to follow-up in ART programmes in low- and middle-income countries: Systematic review and meta-analysis. Trop Med Int Heal. 2015;20:365–379. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 AI031834/AI/NIAID NIH HHS/United States
- UM1 AI069434/AI/NIAID NIH HHS/United States
- U01 AI069923/AI/NIAID NIH HHS/United States
- U01 AI069432/AI/NIAID NIH HHS/United States
- HCP-97105/CIHR/Canada
- R01 DA012568/DA/NIDA NIH HHS/United States
- U01 AI035040/AI/NIAID NIH HHS/United States
- U01 AI034993/AI/NIAID NIH HHS/United States
- M01 RR000083/RR/NCRR NIH HHS/United States
- U01 AI035039/AI/NIAID NIH HHS/United States
- R01 AA016893/AA/NIAAA NIH HHS/United States
- P30 AI027767/AI/NIAID NIH HHS/United States
- U01 AI035042/AI/NIAID NIH HHS/United States
- U01 AI069434/AI/NIAID NIH HHS/United States
- M01 RR000079/RR/NCRR NIH HHS/United States
- K23 AG024896/AG/NIA NIH HHS/United States
- U01 AI037984/AI/NIAID NIH HHS/United States
- R01 DA011602/DA/NIDA NIH HHS/United States
- K23 EY013707/EY/NEI NIH HHS/United States
- K01 AI071725/AI/NIAID NIH HHS/United States
- TGF-96118/CIHR/Canada
- Z01 CP010176/ImNIH/Intramural NIH HHS/United States
- U01 AI096299/AI/NIAID NIH HHS/United States
- U01 AI035004/AI/NIAID NIH HHS/United States
- UM1 AI069432/AI/NIAID NIH HHS/United States
- P30 AI054999/AI/NIAID NIH HHS/United States
- K24 DA000432/DA/NIDA NIH HHS/United States
- CBR-94036/CIHR/Canada
- P30 MH043520/MH/NIMH NIH HHS/United States
- U01 AI069927/AI/NIAID NIH HHS/United States
- U01 AI038855/AI/NIAID NIH HHS/United States
- KRS-86251/CIHR/Canada
- R01 AG029154/AG/NIA NIH HHS/United States
- R01 DA004334/DA/NIDA NIH HHS/United States
- UL1 RR024131/RR/NCRR NIH HHS/United States
- U01 AI034989/AI/NIAID NIH HHS/United States
- 169621 /CIHR/Canada
- U01 AI037613/AI/NIAID NIH HHS/United States
- M01 RR000071/RR/NCRR NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- M01 RR000722/RR/NCRR NIH HHS/United States
- U01 AI035041/AI/NIAID NIH HHS/United States
- R24 AI067039/AI/NIAID NIH HHS/United States
- U01 AI038858/AI/NIAID NIH HHS/United States
- U10 AA013566/AA/NIAAA NIH HHS/United States
- U10 EY008057/EY/NEI NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- U01 AI034994/AI/NIAID NIH HHS/United States
- M01 RR000052/RR/NCRR NIH HHS/United States
- U01 AI069911/AI/NIAID NIH HHS/United States
- U10 EY008052/EY/NEI NIH HHS/United States
- CBR-86906/CIHR/Canada
- P30 AI027763/AI/NIAID NIH HHS/United States
- U01 AI069919/AI/NIAID NIH HHS/United States
- K01 AI093197/AI/NIAID NIH HHS/United States
- U01 AI069918/AI/NIAID NIH HHS/United States
- U01 AI069907/AI/NIAID NIH HHS/United States
- N02 CP055504/CP/NCI NIH HHS/United States
- U01 AI035043/AI/NIAID NIH HHS/United States
- P30 AI027757/AI/NIAID NIH HHS/United States
- UL1 RR025747/RR/NCRR NIH HHS/United States
- U10 EY008067/EY/NEI NIH HHS/United States
- U01 AI069924/AI/NIAID NIH HHS/United States
- U01 AI068634/AI/NIAID NIH HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States
- U01 HD032632/HD/NICHD NIH HHS/United States
- U01 AI042590/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

