Blue-light induced accumulation of reactive oxygen species is a consequence of the Drosophila cryptochrome photocycle
- PMID: 28296892
- PMCID: PMC5351967
- DOI: 10.1371/journal.pone.0171836
Blue-light induced accumulation of reactive oxygen species is a consequence of the Drosophila cryptochrome photocycle
Abstract
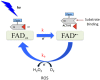
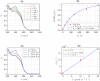
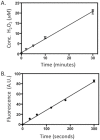
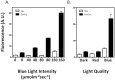
Cryptochromes are evolutionarily conserved blue-light absorbing flavoproteins which participate in many important cellular processes including in entrainment of the circadian clock in plants, Drosophila and humans. Drosophila melanogaster cryptochrome (DmCry) absorbs light through a flavin (FAD) cofactor that undergoes photoreduction to the anionic radical (FAD•-) redox state both in vitro and in vivo. However, recent efforts to link this photoconversion to the initiation of a biological response have remained controversial. Here, we show by kinetic modeling of the DmCry photocycle that the fluence dependence, quantum yield, and half-life of flavin redox state interconversion are consistent with the anionic radical (FAD•-) as the signaling state in vivo. We show by fluorescence detection techniques that illumination of purified DmCry results in enzymatic conversion of molecular oxygen (O2) to reactive oxygen species (ROS). We extend these observations in living cells to demonstrate transient formation of superoxide (O2•-), and accumulation of hydrogen peroxide (H2O2) in the nucleus of insect cell cultures upon DmCry illumination. These results define the kinetic parameters of the Drosophila cryptochrome photocycle and support light-driven electron transfer to the flavin in DmCry signaling. They furthermore raise the intriguing possibility that light-dependent formation of ROS as a byproduct of the cryptochrome photocycle may contribute to its signaling role.
Conflict of interest statement
Figures

References
-
- Chaves I, Pokorny R, Byrdin M, Hoang N, Ritz T, Brettel K, et al. The cryptochromes: blue light photoreceptors in plants and animals. Annual Review in Plant Biology. 2011; 62: 335–364. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

