Longitudinal dynamics of the HIV-specific B cell response during intermittent treatment of primary HIV infection
- PMID: 28296911
- PMCID: PMC5351995
- DOI: 10.1371/journal.pone.0173577
Longitudinal dynamics of the HIV-specific B cell response during intermittent treatment of primary HIV infection
Abstract
Background: Neutralizing antibodies develop in natural HIV-1 infection. Their development often takes several years and may rely on chronic virus exposure. At the same time recent studies show that treatment early in infection may provide opportunities for immune preservation. However, it is unknown how intermittent treatment in early infection affects development of the humoral immune response over time. We investigate the effect of cART in early HIV infection on the properties of the memory B cell compartment following 6 months of cART or in the absence of treatment. The patients included participated in the Primo-SHM trial where patients with an early HIV-1 infection were randomized to no treatment or treatment for 24 or 60 weeks.
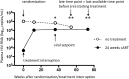
Methods: Primo-SHM trial patients selected for the present study were untreated (n = 23) or treated for 24 weeks (n = 24). Here we investigate memory B cell properties at viral set-point and at a late time point (respectively median 54 and 73 weeks) before (re)-initiation of treatment.
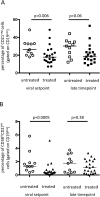
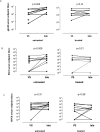
Results: At viral set-point, the memory B cell compartment in treated patients demonstrated significantly lower fractions of antigen-primed, activated, memory B cells (p = 0.006). In contrast to untreated patients, in treated patients the humoral HIV-specific response reached a set point over time. At a transcriptional level, sets of genes that showed enhanced expression in memory B cells at viral setpoint in untreated patients, conversely showed rapid increase of expression of the same genes in treated patients at the late time point.
Conclusion: These data suggest that, although the memory B cell compartment is phenotypically preserved until viral setpoint after treatment interruption, the development of the HIV-specific antibody response may benefit from exposure to HIV. The effect of viral exposure on B cell properties is also reflected by longitudinal changes in transcriptional profile in memory B cells over time in early treated patients.
Conflict of interest statement
Figures



Similar articles
-
A Longitudinal Analysis Reveals Early Activation and Late Alterations in B Cells During Primary HIV Infection in Mozambican Adults.Front Immunol. 2021 Jan 15;11:614319. doi: 10.3389/fimmu.2020.614319. eCollection 2020. Front Immunol. 2021. PMID: 33519823 Free PMC article.
-
A lower viral set point but little immunological impact after early treatment during primary HIV infection.Viral Immunol. 2015 Apr;28(3):134-44. doi: 10.1089/vim.2014.0094. Epub 2015 Mar 6. Viral Immunol. 2015. PMID: 25746670 Free PMC article. Clinical Trial.
-
HIV-Specific B Cell Frequency Correlates with Neutralization Breadth in Patients Naturally Controlling HIV-Infection.EBioMedicine. 2017 Jul;21:158-169. doi: 10.1016/j.ebiom.2017.05.029. Epub 2017 May 31. EBioMedicine. 2017. PMID: 28615147 Free PMC article.
-
Clonal dominance: cause for a limited and failing immune response to HIV-1 infection and vaccination.J Acquir Immune Defic Syndr (1988). 1992;5(11):1158-68. J Acquir Immune Defic Syndr (1988). 1992. PMID: 1403647 Review.
-
B cell immunopathology during HIV-1 infection: lessons to learn for HIV-1 vaccine design.Vaccine. 2008 Jun 6;26(24):3016-25. doi: 10.1016/j.vaccine.2007.11.063. Epub 2007 Dec 17. Vaccine. 2008. PMID: 18164520 Review.
Cited by
-
Advancing HIV Broadly Neutralizing Antibodies: From Discovery to the Clinic.Front Public Health. 2021 May 26;9:690017. doi: 10.3389/fpubh.2021.690017. eCollection 2021. Front Public Health. 2021. PMID: 34123998 Free PMC article. Review.
-
Effective B cell activation in vitro during viremic HIV-1 infection with surrogate T cell stimulation.Immunobiology. 2018 Dec;223(12):839-849. doi: 10.1016/j.imbio.2018.08.007. Epub 2018 Sep 7. Immunobiology. 2018. PMID: 30219203 Free PMC article.
-
Plasmodium-specific atypical memory B cells are short-lived activated B cells.Elife. 2018 Nov 2;7:e39800. doi: 10.7554/eLife.39800. Elife. 2018. PMID: 30387712 Free PMC article.
References
-
- Parren PW, Marx PA, Hessell AJ, Luckay A, Harouse J, Cheng-Mayer C, et al. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J Virol. 2001. September;75(17):8340–7. 10.1128/JVI.75.17.8340-8347.2001 - DOI - PMC - PubMed
-
- Pegu P, Vaccari M, Gordon S, Keele BF, Doster M, Guan Y, et al. Antibodies with high avidity to the gp120 envelope protein in protection from simian immunodeficiency virus SIV(mac251) acquisition in an immunization regimen that mimics the RV-144 Thai trial. J Virol. 2013. February;87(3):1708–19. 10.1128/JVI.02544-12 - DOI - PMC - PubMed
-
- Burton DR, Hessell AJ, Keele BF, Klasse PJ, Ketas TA, Moldt B, et al. Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody. Proc Natl Acad Sci U S A. 2011. July 5;108(27):11181–6. 10.1073/pnas.1103012108 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

