Co-ingestion of carbohydrate and whey protein increases fasted rates of muscle protein synthesis immediately after resistance exercise in rats
- PMID: 28296942
- PMCID: PMC5351968
- DOI: 10.1371/journal.pone.0173809
Co-ingestion of carbohydrate and whey protein increases fasted rates of muscle protein synthesis immediately after resistance exercise in rats
Abstract
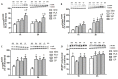
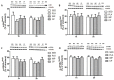
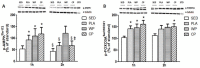
The objective of the study was to investigate whether co-ingestion of carbohydrate and protein as compared with protein alone augments muscle protein synthesis (MPS) during early exercise recovery. Two months old rats performed 10 repetitions of ladder climbing with 75% of body weight attached to their tails. Placebo (PLA), whey protein (WP), or whey protein plus carbohydrate (CP) was then given to rats by gavage. An additional group of sedentary rats (SED) was used as controls. Blood samples were collected immediately and at either 1 or 2 h after exercise. The flexor hallucis longus muscle was excised at 1 or 2 h post exercise for analysis of MPS and related signaling proteins. MPS was significantly increased by CP compared with PLA (p<0.05), and approached significance compared with WP at 1 h post exercise (p = 0.08). CP yielded a greater phosphorylation of mTOR compared with SED and PLA at 1 h post exercise and SED and WP at 2 h post exercise. CP also increased phosphorylation of p70S6K compared with SED at 1 and 2 h post exercise. 4E-BP1 phosphorylation was inhibited by PLA at 1 h but elevated by WP and CP at 2 h post exercise relative to SED. The phosphorylation of AMPK was elevated by exercise at 1 h post exercise, and this elevated level was sustained only in the WP group at 2 h. The phosphorylation of Akt, GSK3, and eIF2Bε were unchanged by treatments. Plasma insulin was transiently increased by CP at 1 h post exercise. In conclusion, post-exercise CP supplementation increases MPS post exercise relative to PLA and possibly WP, which may have been mediated by greater activation of the mTOR signaling pathway.
Conflict of interest statement
Figures

Similar articles
-
Effects of Whey Protein Combined with Amylopectin/Chromium on the Muscle Protein Synthesis and mTOR Phosphorylation in Exercised Rats.Biol Trace Elem Res. 2024 Mar;202(3):1031-1040. doi: 10.1007/s12011-023-03732-x. Epub 2023 Jun 21. Biol Trace Elem Res. 2024. PMID: 37341874
-
Co-ingestion of carbohydrate and whey protein induces muscle strength and myofibrillar protein accretion without a requirement of satellite cell activation.Curr Res Physiol. 2020 Feb 11;2:12-21. doi: 10.1016/j.crphys.2020.02.001. eCollection 2020 Jun. Curr Res Physiol. 2020. PMID: 34746812 Free PMC article.
-
L-Alanylglutamine inhibits signaling proteins that activate protein degradation, but does not affect proteins that activate protein synthesis after an acute resistance exercise.Amino Acids. 2015 Jul;47(7):1389-98. doi: 10.1007/s00726-015-1972-7. Epub 2015 Apr 3. Amino Acids. 2015. PMID: 25837301
-
Whey protein but not collagen peptides stimulate acute and longer-term muscle protein synthesis with and without resistance exercise in healthy older women: a randomized controlled trial.Am J Clin Nutr. 2020 Mar 1;111(3):708-718. doi: 10.1093/ajcn/nqz332. Am J Clin Nutr. 2020. PMID: 31919527 Free PMC article. Clinical Trial.
-
International society of sports nutrition position stand: nutrient timing.J Int Soc Sports Nutr. 2017 Aug 29;14:33. doi: 10.1186/s12970-017-0189-4. eCollection 2017. J Int Soc Sports Nutr. 2017. PMID: 28919842 Free PMC article. Review.
Cited by
-
Effects of Whey Protein Combined with Amylopectin/Chromium on the Muscle Protein Synthesis and mTOR Phosphorylation in Exercised Rats.Biol Trace Elem Res. 2024 Mar;202(3):1031-1040. doi: 10.1007/s12011-023-03732-x. Epub 2023 Jun 21. Biol Trace Elem Res. 2024. PMID: 37341874
-
Seaweed Supplementation Enhances Maximal Muscular Strength and Attenuates Resistance Exercise-Induced Oxidative Stress in Rats.Evid Based Complement Alternat Med. 2019 Jul 28;2019:3528932. doi: 10.1155/2019/3528932. eCollection 2019. Evid Based Complement Alternat Med. 2019. PMID: 31467574 Free PMC article.
-
The addition of an amylopectin/chromium complex to branched-chain amino acids enhances muscle protein synthesis in rat skeletal muscle.J Int Soc Sports Nutr. 2020 May 27;17(1):26. doi: 10.1186/s12970-020-00355-8. J Int Soc Sports Nutr. 2020. PMID: 32460884 Free PMC article.
-
Low-fat, lactose-free and leucine-enriched chocolate cow milk prototype: A preliminary study on sensorial acceptability and gastrointestinal complaints following exhaustive exercise.J Int Soc Sports Nutr. 2021 Feb 10;18(1):14. doi: 10.1186/s12970-020-00406-0. J Int Soc Sports Nutr. 2021. PMID: 33568169 Free PMC article.
-
Co-ingestion of carbohydrate and whey protein induces muscle strength and myofibrillar protein accretion without a requirement of satellite cell activation.Curr Res Physiol. 2020 Feb 11;2:12-21. doi: 10.1016/j.crphys.2020.02.001. eCollection 2020 Jun. Curr Res Physiol. 2020. PMID: 34746812 Free PMC article.
References
-
- Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol. 1997;273(1 Pt 1):E99–107. Epub 1997/07/01. - PubMed
-
- Hernandez JM, Fedele MJ, Farrell PA. Time course evaluation of protein synthesis and glucose uptake after acute resistance exercise in rats. J Appl Physiol. 2000;88(3):1142–9. Epub 2000/03/10. - PubMed
-
- Anthony TG, McDaniel BJ, Knoll P, Bunpo P, Paul GL, McNurlan MA. Feeding meals containing soy or whey protein after exercise stimulates protein synthesis and translation initiation in the skeletal muscle of male rats. J Nutr. 2007;137(2):357–62. Epub 2007/01/24. - PubMed
-
- Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol. 1995;268(3 Pt 1):E514–20. Epub 1995/03/01. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

