Estradiol alters the immune-responsiveness of cervical epithelial cells stimulated with ligands of Toll-like receptors 2 and 4
- PMID: 28296959
- PMCID: PMC5351915
- DOI: 10.1371/journal.pone.0173646
Estradiol alters the immune-responsiveness of cervical epithelial cells stimulated with ligands of Toll-like receptors 2 and 4
Abstract
The mucosa of the female reproductive tract plays a pivotal role in host defence. Pregnancy must alter immunological mechanisms at this interface to protect the conceptus. We sought to determine how estradiol (E2) alters the immune-responsiveness of cervical epithelial cells to ligand stimulation of Toll-like receptor (TLR)-2 and -4. Human ectocervical epithelial cells (HECECs) were cultured and co-incubated with two concentrations of E2 and peptidoglycan (PGN) or lipopolysaccharide (LPS) over durations that ranged between 10 minutes and 18 hours. Cytometric Bead Array was performed to quantify eight cytokines in the supernatant fluid. In response to PGN, HECECs co-incubated with E2 released lesser quantities of IL-1ß and IFNγ, higher levels of RANTES, and variable levels of IL-6 and IL-8 than those not exposed to E2. In contrast, HECECs co-incubated with LPS and E2 secreted increased levels of IL-1ß, IL-6, IL-8, and IFNγ at 2 and 18 hours than HECECs not exposed to E2, and reduced levels of RANTES at same study time-points. Estradiol alters the immune-responsiveness of cultured HECECs to TLR2 and TLR4 ligands in a complex fashion that appears to vary with bacterial ligand, TLR subtype, and duration of exposure. Our observations are consistent with the functional complexity that this mucosal interface requires for its immunological roles.
Conflict of interest statement
Figures
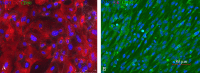

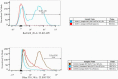
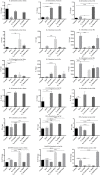
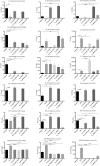
References
-
- Hickey DK, Patel M V., Fahey J V., Wira CR. Innate and adaptive immunity at mucosal surfaces of the female reproductive tract: Stratification and integration of immune protection against the transmission of sexually transmitted infections. Journal of Reproductive Immunology. 2011. pp. 185–194. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

