Human Lung Spheroids as In Vitro Niches of Lung Progenitor Cells with Distinctive Paracrine and Plasticity Properties
- PMID: 28297570
- PMCID: PMC5442776
- DOI: 10.5966/sctm.2015-0374
Human Lung Spheroids as In Vitro Niches of Lung Progenitor Cells with Distinctive Paracrine and Plasticity Properties
Abstract
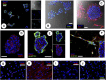
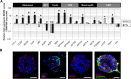
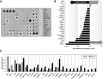
Basic and translational research on lung biology has discovered multiple progenitor cell types, specialized or facultative, responsible for turnover, renewal, and repair. Isolation of populations of resident lung progenitor cells (LPCs) has been described by multiple protocols, and some have been successfully applied to healthy human lung tissue. We aimed at understanding how different cell culture conditions may affect, in vitro, the phenotype of LPCs to create an ideal niche-like microenvironment. The influence of different substrates (i.e., fibronectin, gelatin, laminin) and the impact of a three-dimensional/two-dimensional (3D/2D) culture switch on the biology of LPCs isolated as lung spheroids (LSs) from normal adult human lung biopsy specimens were investigated. We applied a spheroid culture system as the selective/inductive step for progenitor cell culture, as described in many biological systems. The data showed a niche-like proepithelial microenvironment inside the LS, highly sensitive to the 3D culture system and significantly affecting the phenotype of adult LPCs more than culture substrate. LSs favor epithelial phenotypes and LPC maintenance and contain cells more responsive to specific commitment stimuli than 2D monolayer cultures, while secreting a distinctive set of paracrine factors. We have shown for the first time, to our knowledge, how culture as 3D LSs can affect LPC epithelial phenotype and produce strong paracrine signals with a distinctive secretomic profile compared with 2D monolayer conditions. These findings suggest novel approaches to maintain ex vivo LPCs for basic and translational studies. Stem Cells Translational Medicine 2017;6:767-777.
Keywords: Epithelial-to-mesenchymal transition; Lung stem cells; Pneumospheres; Stem cell niche; Three-dimensional culture.
© 2016 The Authors Stem Cells Translational Medicine published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Figures


References
-
- European Respiratory Society. The burden of lung disease. Available at http://www.erswhitebook.org/chapters/the‐burden‐of‐lung‐disease/. Accessed September 18, 2016.
-
- World Health Organization. World Health Statistics 2011. Available at http://www.who.int/whosis/whostat/2011/en. Accessed November 30, 2015.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

