Magnetic Resonance Imaging-Guided Delivery of Neural Stem Cells into the Basal Ganglia of Nonhuman Primates Reveals a Pulsatile Mode of Cell Dispersion
- PMID: 28297573
- PMCID: PMC5442780
- DOI: 10.5966/sctm.2016-0269
Magnetic Resonance Imaging-Guided Delivery of Neural Stem Cells into the Basal Ganglia of Nonhuman Primates Reveals a Pulsatile Mode of Cell Dispersion
Abstract
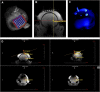
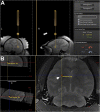
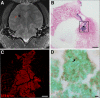
Optimal stem cell delivery procedures are critical to the success of the cell therapy approach. Variables such as flow rate, suspension solution, needle diameter, cell density, and tissue mechanics affect tissue penetration, backflow along the needle, and the dispersion and survival of injected cells during delivery. Most cell transplantation centers engaged in human clinical trials use custom-designed cannula needles, syringes, or catheters, sometimes precluding the use of magnetic resonance imaging (MRI)-guided delivery to target tissue. As a result, stem cell therapies may be hampered because more than 80% of grafted cells do not survive the delivery-for example, to the heart, liver/pancreas, and brain-which translates to poor patient outcomes. We developed a minimally invasive interventional MRI (iMRI) approach for intraoperatively imaging neural stem cell (NSC) delivery procedures. We used NSCs prelabeled with a contrast agent and real-time magnetic resonance imaging to guide the injection cannula to the target and to track the delivery of the cells into the putamen of baboons. We provide evidence that cell injection into the brain parenchyma follows a novel pulsatile mode of cellular discharge from the delivery catheter despite a constant infusion flow rate. The rate of cell infusion significantly affects the dispersion and viability of grafted cells. We report on our investigational use of a frameless navigation system for image-guided NSC transplantation using a straight cannula. Through submillimeter accuracy and real-time imaging, iMRI approaches may improve the safety and efficacy of neural cell transplantation therapies. Stem Cells Translational Medicine 2017;6:877-885.
Keywords: Cell flow; ClearPoint system; Interventional magnetic resonance imaging; Nonhuman primate; Real-time interventional magnetic resonance imaging-guided cell transplantation; Rheology; Stem cell delivery.
© 2016 The Authors Stem Cells Translational Medicine published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Figures


References
-
- Shapiro AM, Geng Hao E, Lakey JR et al. Defining optimal immunosuppression for islet transplantation based on reduced diabetogenicity in canine islet autografts. Transplantation 2002;74:1522–1528. - PubMed
-
- Hagell P, Brundin P. Cell survival and clinical outcome following intrastriatal transplantation in Parkinson disease. J Neuropathol Exp Neurol 2001;60:741–752. - PubMed
-
- Daadi MM, Pivirotto P, Bringas J et al. Distribution of AAV2‐hAADC‐transduced cells after 3 years in Parkinsonian monkeys. Neuroreport 2006;17:201–204. - PubMed
-
- Bankiewicz K, Daadi M, Pivirotto P et al. Focal dopamine production in the striatum potentiaties dyskinesias in non‐human primate model of Parkinson’s disease and may explain induction of run‐away dyskinesias in PD patients after fetal mesencephalic implantation. Neurology 2004;62:A11–A12.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

