Oncostatin M-Preconditioned Mesenchymal Stem Cells Alleviate Bleomycin-Induced Pulmonary Fibrosis Through Paracrine Effects of the Hepatocyte Growth Factor
- PMID: 28297588
- PMCID: PMC5442768
- DOI: 10.5966/sctm.2016-0054
Oncostatin M-Preconditioned Mesenchymal Stem Cells Alleviate Bleomycin-Induced Pulmonary Fibrosis Through Paracrine Effects of the Hepatocyte Growth Factor
Abstract
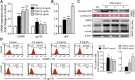
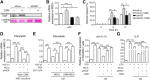
Mesenchymal stem cells (MSCs) are widely considered for treatment of pulmonary fibrosis based on the anti-inflammatory, antifibrotic, antiapoptotic, and regenerative properties of the cells. Recently, elevated levels of oncostatin M (OSM) have been reported in the bronchoalveolar lavage fluid of a pulmonary fibrosis animal model and in patients. In this work, we aimed to prolong engrafted MSC survival and to enhance the effectiveness of pulmonary fibrosis transplantation therapy by using OSM-preconditioned MSCs. OSM-preconditioned MSCs were shown to overexpress type 2 OSM receptor (gp130/OSMRβ) and exhibited high susceptibility to OSM, resulting in upregulation of the paracrine factor, hepatocyte growth factor (HGF). Moreover, OSM-preconditioned MSCs enhanced cell proliferation and migration, attenuated transforming growth factor-β1- or OSM-induced extracellular matrix production in MRC-5 fibroblasts through paracrine effects. In bleomycin-induced lung fibrotic mice, transplantation of OSM-preconditioned MSCs significantly improved pulmonary respiratory functions and downregulated expression of inflammatory factors and fibrotic factors in the lung tissues. Histopathologic examination indicated remarkable amelioration of the lung fibrosis. LacZ-tagged MSCs were detected in the lung tissues of the OSM-preconditioned MSC-treated mice 18 days after post-transplantation. Taken together, our data further demonstrated that HGF upregulation played an important role in mediating the therapeutic effects of transplanted OSM-preconditioned MSCs in alleviating lung fibrosis in the mice. Stem Cells Translational Medicine 2017;6:1006-1017.
Keywords: Bleomycin-induced pulmonary fibrosis; Hepatocyte growth factor; Oncostatin M; Preconditioning; Stem cells.
© 2016 The Authors Stem Cells Translational Medicine published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Figures




References
-
- Spagnolo P. Novel treatments for idiopathic pulmonary fibrosis. Am J Med 2015;128:447–449. - PubMed
-
- Antunes MA, Laffey JG, Pelosi P et al. Mesenchymal stem cell trials for pulmonary diseases. J Cell Biochem 2014;115:1023–1032. - PubMed
-
- Glassberg MK, Toonkel RL. Moving stem cell therapy to patients with idiopathic pulmonary fibrosis. Respirology 2014;19:950–951. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

