Phosphorylation-Induced Mechanical Regulation of Intrinsically Disordered Neurofilament Proteins
- PMID: 28297648
- PMCID: PMC5355543
- DOI: 10.1016/j.bpj.2016.12.050
Phosphorylation-Induced Mechanical Regulation of Intrinsically Disordered Neurofilament Proteins
Abstract
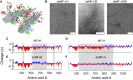
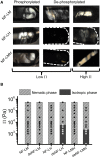
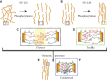
The biological function of protein assemblies has been conventionally equated with a unique three-dimensional protein structure and protein-specific interactions. However, in the past 20 years it has been found that some assemblies contain long flexible regions that adopt multiple structural conformations. These include neurofilament proteins that constitute the stress-responsive supportive network of neurons. Herein, we show that the macroscopic properties of neurofilament networks are tuned by enzymatic regulation of the charge found on the flexible protein regions. The results reveal an enzymatic (phosphorylation) regulation of macroscopic properties such as orientation, stress response, and expansion in flexible protein assemblies. Using a model that explains the attractive electrostatic interactions induced by enzymatically added charges, we demonstrate that phosphorylation regulation is far richer and versatile than previously considered.
Copyright © 2017 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
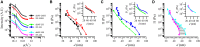
Similar articles
-
Structural Regulation of a Neurofilament-Inspired Intrinsically Disordered Protein Brush by Multisite Phosphorylation.Biochemistry. 2018 Jul 10;57(27):4019-4028. doi: 10.1021/acs.biochem.8b00007. Epub 2018 Apr 6. Biochemistry. 2018. PMID: 29557644
-
Folding of an intrinsically disordered protein by phosphorylation as a regulatory switch.Nature. 2015 Mar 5;519(7541):106-9. doi: 10.1038/nature13999. Epub 2014 Dec 22. Nature. 2015. PMID: 25533957
-
The Effect of Multisite Phosphorylation on the Conformational Properties of Intrinsically Disordered Proteins.Int J Mol Sci. 2021 Oct 14;22(20):11058. doi: 10.3390/ijms222011058. Int J Mol Sci. 2021. PMID: 34681718 Free PMC article.
-
Structures and interactions in 'bottlebrush' neurofilaments: the role of charged disordered proteins in forming hydrogel networks.Biochem Soc Trans. 2012 Oct;40(5):1027-31. doi: 10.1042/BST20120101. Biochem Soc Trans. 2012. PMID: 22988859 Review.
-
Simulations of disordered proteins and systems with conformational heterogeneity.Curr Opin Struct Biol. 2017 Apr;43:95-103. doi: 10.1016/j.sbi.2016.11.006. Epub 2016 Dec 15. Curr Opin Struct Biol. 2017. PMID: 27988422 Review.
Cited by
-
Phosphorylation of disordered proteins tunes local and global intramolecular interactions.bioRxiv [Preprint]. 2024 Jun 12:2024.06.10.598315. doi: 10.1101/2024.06.10.598315. bioRxiv. 2024. Update in: Biophys J. 2024 Dec 3;123(23):4082-4096. doi: 10.1016/j.bpj.2024.10.021. PMID: 38915510 Free PMC article. Updated. Preprint.
-
From isolated polyelectrolytes to star-like assemblies: the role of sequence heterogeneity on the statistical structure of the intrinsically disordered neurofilament-low tail domain.Eur Phys J E Soft Matter. 2024 Feb 15;47(2):13. doi: 10.1140/epje/s10189-024-00409-8. Eur Phys J E Soft Matter. 2024. PMID: 38358563 Free PMC article.
-
Order from Disorder with Intrinsically Disordered Peptide Amphiphiles.J Am Chem Soc. 2021 Aug 4;143(30):11879-11888. doi: 10.1021/jacs.1c06133. Epub 2021 Jul 26. J Am Chem Soc. 2021. PMID: 34310121 Free PMC article.
-
Neurofilament Biophysics: From Structure to Biomechanics.Mol Biol Cell. 2024 May 1;35(5):re1. doi: 10.1091/mbc.E23-11-0438. Epub 2024 Apr 10. Mol Biol Cell. 2024. PMID: 38598299 Free PMC article. Review.
-
Phosphorylation of disordered proteins tunes local and global intramolecular interactions.Biophys J. 2024 Dec 3;123(23):4082-4096. doi: 10.1016/j.bpj.2024.10.021. Epub 2024 Nov 13. Biophys J. 2024. PMID: 39539017 Free PMC article.
References
-
- Uversky V.N. Intrinsically disordered proteins from A to Z. Int. J. Biochem. Cell Biol. 2011;43:1090–1103. - PubMed
-
- Chen L., Feany M.B. α-Synuclein phosphorylation controls neurotoxicity and inclusion formation in a Drosophila model of Parkinson disease. Nat. Neurosci. 2005;8:657–663. - PubMed
-
- Stoothoff W.H., Johnson G.V.W. Tau phosphorylation: physiological and pathological consequences. Biochim. Biophys. Acta. 2005;1739:280–297. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

