Glycan Determinants of Heparin-Tau Interaction
- PMID: 28297651
- PMCID: PMC5355497
- DOI: 10.1016/j.bpj.2017.01.024
Glycan Determinants of Heparin-Tau Interaction
Abstract
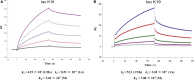
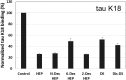
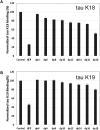
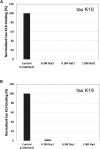
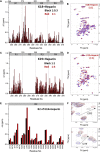
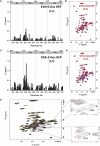
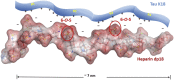
Tau aggregates into paired helical filaments within neurons, a pathological hallmark of Alzheimer's disease. Heparin promotes tau aggregation and recently has been shown to be involved in the cellular uptake of tau aggregates. Although the tau-heparin interaction has been extensively studied, little is known about the glycan determinants of this interaction. Here, we used surface plasmon resonance (SPR) and NMR spectroscopy to characterize the interaction between two tau fragments, K18 and K19, and several polysaccharides, including heparin, heparin oligosaccharides, chemically modified heparin, and related glycans. Using a heparin-immobilized chip, SPR revealed that tau K18 and K19 bind heparin with a KD of 0.2 and 70 μM, respectively. In SPR competition experiments, N-desulfation and 2-O-desulfation had no effect on heparin binding to K18, whereas 6-O-desulfation severely reduced binding, suggesting a critical role for 6-O-sulfation in the tau-heparin interaction. The tau-heparin interaction became stronger with longer-chain heparin oligosaccharides. As expected for an electrostatics-driven interaction, a moderate amount of salt (0.3 M NaCl) abolished binding. NMR showed the largest chemical-shift perturbation (CSP) in R2 in tau K18, which was absent in K19, revealing differential binding sites in K18 and K19 to heparin. Dermatan sulfate binding produced minimal CSP, whereas dermatan disulfate, with the additional 6-O-sulfo group, induced much larger CSP. 2-O-desulfated heparin induced much larger CSP in K18 than 6-O-desulfated heparin. Our data demonstrate a crucial role for the 6-O-sulfo group in the tau-heparin interaction, which to our knowledge has not been reported before.
Copyright © 2017 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Schoenfeld T.A., Obar R.A. Diverse distribution and function of fibrous microtubule-associated proteins in the nervous system. Int. Rev. Cytol. 1994;151:67–137. - PubMed
-
- Trojanowski J.Q., Lee V.M. Pathological tau: a loss of normal function or a gain in toxicity? Nat. Neurosci. 2005;8:1136–1137. - PubMed
-
- Iqbal K., Alonso Adel. C., Grundke-Iqba I. Alzheimer neurofibrillary degeneration: therapeutic targets and high-throughput assays. J. Mol. Neurosci. 2003;20:425–429. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

