High-Throughput Patch Clamp Screening in Human α6-Containing Nicotinic Acetylcholine Receptors
- PMID: 28298165
- PMCID: PMC5480602
- DOI: 10.1177/2472555217696794
High-Throughput Patch Clamp Screening in Human α6-Containing Nicotinic Acetylcholine Receptors
Abstract
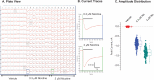
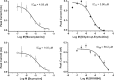
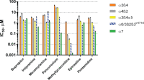
Nicotine, the addictive component of tobacco products, is an agonist at nicotinic acetylcholine receptors (nAChRs) in the brain. The subtypes of nAChR are defined by their α- and β-subunit composition. The α6β2β3 nAChR subtype is expressed in terminals of dopaminergic neurons that project to the nucleus accumbens and striatum and modulate dopamine release in brain regions involved in nicotine addiction. Although subtype-dependent selectivity of nicotine is well documented, subtype-selective profiles of other tobacco product constituents are largely unknown and could be essential for understanding the addiction-related neurological effects of tobacco products. We describe the development and validation of a recombinant cell line expressing human α6/3β2β3V273S nAChR for screening and profiling assays in an automated patch clamp platform (IonWorks Barracuda). The cell line was pharmacologically characterized by subtype-selective and nonselective reference agonists, pore blockers, and competitive antagonists. Agonist and antagonist effects detected by the automated patch clamp approach were comparable to those obtained by conventional electrophysiological assays. A pilot screen of a library of Food and Drug Administration-approved drugs identified compounds, previously not known to modulate nAChRs, which selectively inhibited the α6/3β2β3V273S subtype. These assays provide new tools for screening and subtype-selective profiling of compounds that act at α6β2β3 nicotinic receptors.
Keywords: automated patch clamp; electrophysiological screening; ion channel; nicotinic acetylcholine receptor.
Conflict of interest statement
Figures

Similar articles
-
Stable expression and functional characterization of a human nicotinic acetylcholine receptor with α6β2 properties: discovery of selective antagonists.Br J Pharmacol. 2011 May;163(2):313-29. doi: 10.1111/j.1476-5381.2011.01213.x. Br J Pharmacol. 2011. PMID: 21232042 Free PMC article.
-
Electrophysiology-Based Assays to Detect Subtype-Selective Modulation of Human Nicotinic Acetylcholine Receptors.Assay Drug Dev Technol. 2016 Aug;14(6):333-44. doi: 10.1089/adt.2015.688. Assay Drug Dev Technol. 2016. PMID: 27505073 Free PMC article.
-
α6-Containing nicotinic acetylcholine receptors in midbrain dopamine neurons are poised to govern dopamine-mediated behaviors and synaptic plasticity.Neuroscience. 2015 Sep 24;304:161-75. doi: 10.1016/j.neuroscience.2015.07.052. Epub 2015 Jul 23. Neuroscience. 2015. PMID: 26210579 Free PMC article.
-
Role of α6 nicotinic receptors in CNS dopaminergic function: relevance to addiction and neurological disorders.Biochem Pharmacol. 2011 Oct 15;82(8):873-82. doi: 10.1016/j.bcp.2011.06.001. Epub 2011 Jun 13. Biochem Pharmacol. 2011. PMID: 21684266 Free PMC article. Review.
-
Mysterious alpha6-containing nAChRs: function, pharmacology, and pathophysiology.Acta Pharmacol Sin. 2009 Jun;30(6):740-51. doi: 10.1038/aps.2009.63. Acta Pharmacol Sin. 2009. PMID: 19498417 Free PMC article. Review.
Cited by
-
Ribbon α-Conotoxin KTM Exhibits Potent Inhibition of Nicotinic Acetylcholine Receptors.Mar Drugs. 2019 Nov 28;17(12):669. doi: 10.3390/md17120669. Mar Drugs. 2019. PMID: 31795126 Free PMC article.
-
High-throughput cell-based assays for identifying antagonists of multiple smoking-associated human nicotinic acetylcholine receptor subtypes.SLAS Discov. 2022 Jan;27(1):68-76. doi: 10.1016/j.slasd.2021.10.001. Epub 2021 Oct 9. SLAS Discov. 2022. PMID: 35058178 Free PMC article.
-
Modulation of cardiomyocyte contractility and action potentials with chemogenetic chloride currents.J Physiol. 2025 Mar;603(6):1399-1415. doi: 10.1113/JP286428. Epub 2025 Feb 24. J Physiol. 2025. PMID: 39992007 Free PMC article.
-
Qualitative Assay to Detect Dopamine Release by Ligand Action on Nicotinic Acetylcholine Receptors.Toxins (Basel). 2019 Nov 20;11(12):682. doi: 10.3390/toxins11120682. Toxins (Basel). 2019. PMID: 31757080 Free PMC article.
References
-
- Dani J. A., Heinemann S. Molecular and Cellular Aspects of Nicotine Abuse. Neuron 1996, 16, 905–908. - PubMed
-
- Coe J. W., Brooks P. R., Vetelino M. G., et al. Varenicline: An Alpha4beta2 Nicotinic Receptor Partial Agonist for Smoking Cessation. J. Med. Chem. 2005, 48, 3474–3477. - PubMed
-
- Wallace T. L., Porter R. H. Targeting the Nicotinic Alpha7 Acetylcholine Receptor to Enhance Cognition in Disease. Biochem. Pharmacol. 2011, 82, 891–903. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

