Concussion Induces Hippocampal Circuitry Disruption in Swine
- PMID: 28298170
- PMCID: PMC5510797
- DOI: 10.1089/neu.2016.4848
Concussion Induces Hippocampal Circuitry Disruption in Swine
Abstract
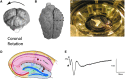
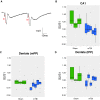
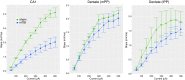
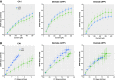
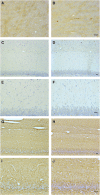
Hippocampal-dependent deficits in learning and memory formation are a prominent feature of traumatic brain injury (TBI); however, the role of the hippocampus in cognitive dysfunction after concussion (mild TBI) is unknown. We therefore investigated functional and structural changes in the swine hippocampus following TBI using a model of head rotational acceleration that closely replicates the biomechanics and neuropathology of closed-head TBI in humans. We examined neurophysiological changes using a novel ex vivo hippocampal slice paradigm with extracellular stimulation and recording in the dentate gyrus and CA1 occurring at 7 days following non-impact inertial TBI in swine. Hippocampal neurophysiology post-injury revealed reduced axonal function, synaptic dysfunction, and regional hyperexcitability at one week following even "mild" injury levels. Moreover, these neurophysiological changes occurred in the apparent absence of intra-hippocampal neuronal or axonal degeneration. Input-output curves demonstrated an elevated excitatory post-synaptic potential (EPSP) output for a given fiber volley input in injured versus sham animals, suggesting a form of homeostatic plasticity that manifested as a compensatory response to decreased axonal function in post-synaptic regions. These data indicate that closed-head rotational acceleration-induced TBI, the common cause of concussion in humans, may induce significant alterations in hippocampal circuitry function that have not resolved at 7 days post-injury. This circuitry dysfunction may underlie some of the post-concussion symptomatology associated with the hippocampus, such as post-traumatic amnesia and ongoing cognitive deficits.
Keywords: axonal pathology; concussion; epileptogenesis; hippocampus; mild TBI; traumatic brain injury.
Conflict of interest statement
No competing financial interests exist.
Figures
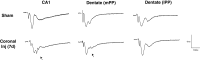
Similar articles
-
Mossy cell hypertrophy and synaptic changes in the hilus following mild diffuse traumatic brain injury in pigs.J Neuroinflammation. 2020 Jan 31;17(1):44. doi: 10.1186/s12974-020-1720-0. J Neuroinflammation. 2020. PMID: 32005260 Free PMC article.
-
Hippocampal interneuronal dysfunction and hyperexcitability in a porcine model of concussion.Commun Biol. 2023 Nov 9;6(1):1136. doi: 10.1038/s42003-023-05491-w. Commun Biol. 2023. PMID: 37945934 Free PMC article.
-
Concussive brain trauma in the mouse results in acute cognitive deficits and sustained impairment of axonal function.J Neurotrauma. 2011 Apr;28(4):547-63. doi: 10.1089/neu.2010.1729. J Neurotrauma. 2011. PMID: 21299360 Free PMC article.
-
The neurophysiology of concussion.Prog Neurobiol. 2002 Jul;67(4):281-344. doi: 10.1016/s0301-0082(02)00018-7. Prog Neurobiol. 2002. PMID: 12207973 Review.
-
[Mild traumatic brain injury and postconcussive syndrome: a re-emergent questioning].Encephale. 2012 Sep;38(4):329-35. doi: 10.1016/j.encep.2011.07.003. Epub 2011 Aug 31. Encephale. 2012. PMID: 22980474 Review. French.
Cited by
-
Gestational dexamethasone exposure impacts hippocampal excitatory synaptic transmission and learning and memory function with transgenerational effects.Acta Pharm Sin B. 2023 Sep;13(9):3708-3727. doi: 10.1016/j.apsb.2023.05.013. Epub 2023 May 15. Acta Pharm Sin B. 2023. PMID: 37719378 Free PMC article.
-
Sex differences in the extent of acute axonal pathologies after experimental concussion.Acta Neuropathol. 2024 May 5;147(1):79. doi: 10.1007/s00401-024-02735-9. Acta Neuropathol. 2024. PMID: 38705966 Free PMC article.
-
A Porcine Model of Traumatic Brain Injury via Head Rotational Acceleration.Methods Mol Biol. 2016;1462:289-324. doi: 10.1007/978-1-4939-3816-2_17. Methods Mol Biol. 2016. PMID: 27604725 Free PMC article.
-
The Presence of the Temporal Horn Exacerbates the Vulnerability of Hippocampus During Head Impacts.Front Bioeng Biotechnol. 2022 Mar 22;10:754344. doi: 10.3389/fbioe.2022.754344. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35392406 Free PMC article.
-
Traumatic Brain Injury Preserves Firing Rates But Disrupts Laminar Oscillatory Coupling and Neuronal Entrainment in Hippocampal CA1.eNeuro. 2020 Sep 2;7(5):ENEURO.0495-19.2020. doi: 10.1523/ENEURO.0495-19.2020. Print 2020 Sep/Oct. eNeuro. 2020. PMID: 32737188 Free PMC article.
References
-
- Coronado V.G., McGuire L.C., Sarmiento K., Bell J., Lionbarger M.R., Jones C.D., Geller A.I., Khoury N., and Xu L. (2012). Trends in traumatic brain injury in the U.S. and the public health response: 1995–2009. J. Safety Res. 43, 299–307 - PubMed
-
- Graham R., Rivara F.P., Ford M.A., and Spicer C.M. (2014). Sports-Related Concussions in Youth: Improving the Science, Changing the Culture. The National Academies Press: Washington, D.C. - PubMed
-
- Jordan B.D. (2013). The clinical spectrum of sport-related traumatic brain injury. Nature reviews. Neurology 9, 222–230 - PubMed
-
- Hoge C.W., McGurk D., Thomas J.L., Cox A.L., Engel C.C., and Castro C.A. (2008). Mild traumatic brain injury in U.S. soldiers returning from Iraq. N. Engl. J. Med. 358, 453–463 - PubMed
-
- Capruso D.X. and Levin H.S. (1992). Cognitive impairment following closed head injury. Neurol. Clin. 10, 879–893 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

