Elutriated lymphocytes for manufacturing chimeric antigen receptor T cells
- PMID: 28298232
- PMCID: PMC5353875
- DOI: 10.1186/s12967-017-1160-5
Elutriated lymphocytes for manufacturing chimeric antigen receptor T cells
Abstract
Background: Clinical trials of Chimeric Antigen Receptor (CAR) T cells manufactured from autologous peripheral blood mononuclear cell (PBMC) concentrates for the treatment of hematologic malignancies have been promising, but CAR T cell yields have been variable. This variability is due in part to the contamination of the PBMC concentrates with monocytes and granulocytes.
Methods: Counter-flow elutriation allows for the closed system separation of lymphocytes from monocytes and granulocytes. We investigated the use of PBMC concentrates enriched for lymphocytes using elutriation for manufacturing 8 CD19- and 5 GD2-CAR T cell products.
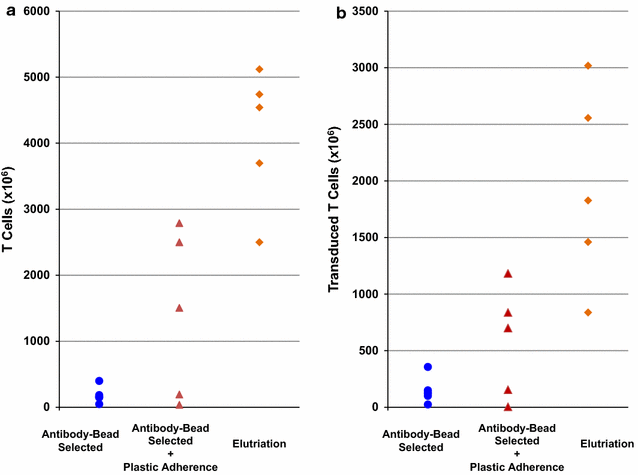
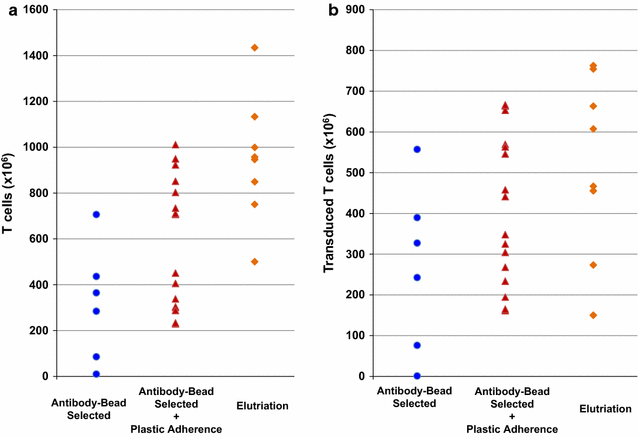
Results: When compared to PBMC concentrates, lymphocyte-enriched elutriation fractions contained greater proportions of CD3+ and CD56+ cells and reduced proportions of CD14+ and CD15+ cells. All 13 CAR T cell products manufactured using the elutriated lymphocytes yielded sufficient quantities of transduced CAR T cells to meet clinical dose criteria. The GD2-CAR T cell products contained significantly more T cells and transduced T cells than the CD19-CAR T cell products. A comparison of the yields of CAR T cells produced from elutriated lymphocytes with the yields of CAR T cells previous produced from cells isolated from PBMC concentrates by anti-CD3/CD28 bead selection or by anti-CD3/CD28 bead selection plus plastic adherence found that greater quantities of GD2-CAR T cells were produced from elutriated lymphocytes, but not CD19-CAR T cells.
Conclusions: Enrichment of PBMC concentrates for lymphocytes using elutriation increased the quantity of GD2-CAR T cells produced. These results provide further evidence that CAR T cell expansion is inhibited by monocytes and granulocytes.
Keywords: Cancer immunotherapy; Cellular therapy; Chimeric antigen receptor T cells; Elutriation; Myeloid derived suppressor cells; Peripheral blood mononuclear cells; T cells.
Figures
Similar articles
-
Myeloid cells in peripheral blood mononuclear cell concentrates inhibit the expansion of chimeric antigen receptor T cells.Cytotherapy. 2016 Jul;18(7):893-901. doi: 10.1016/j.jcyt.2016.04.003. Epub 2016 May 17. Cytotherapy. 2016. PMID: 27210719 Free PMC article. Clinical Trial.
-
Counter-flow elutriation of clinical peripheral blood mononuclear cell concentrates for the production of dendritic and T cell therapies.J Transl Med. 2014 Sep 17;12:241. doi: 10.1186/s12967-014-0241-y. J Transl Med. 2014. PMID: 25223845 Free PMC article.
-
Effects of starting cellular material composition on chimeric antigen receptor T-cell expansion and characteristics.Transfusion. 2019 May;59(5):1755-1764. doi: 10.1111/trf.15287. Epub 2019 Apr 11. Transfusion. 2019. PMID: 30973976
-
Incorporation of Immune Checkpoint Blockade into Chimeric Antigen Receptor T Cells (CAR-Ts): Combination or Built-In CAR-T.Int J Mol Sci. 2018 Jan 24;19(2):340. doi: 10.3390/ijms19020340. Int J Mol Sci. 2018. PMID: 29364163 Free PMC article. Review.
-
[Prerequisite for hematopoietic cellular therapy programs to set up chimeric antigen receptor T-cell therapy (CAR T-cells): Guidelines from the Francophone Society of Bone Marrow Transplantation and Cellular Therapy (SFGM-TC)].Bull Cancer. 2017 Dec;104(12S):S43-S58. doi: 10.1016/j.bulcan.2017.10.017. Epub 2017 Nov 23. Bull Cancer. 2017. PMID: 29174320 French.
Cited by
-
Depletion of high-content CD14+ cells from apheresis products is critical for successful transduction and expansion of CAR T cells during large-scale cGMP manufacturing.Mol Ther Methods Clin Dev. 2021 Jul 16;22:377-387. doi: 10.1016/j.omtm.2021.06.014. eCollection 2021 Sep 10. Mol Ther Methods Clin Dev. 2021. PMID: 34514029 Free PMC article.
-
Mechanisms of resistance to CAR T cell therapies.Semin Cancer Biol. 2020 Oct;65:91-98. doi: 10.1016/j.semcancer.2019.12.002. Epub 2019 Dec 19. Semin Cancer Biol. 2020. PMID: 31866478 Free PMC article. Review.
-
Improved expansion of T cells in culture when isolated with an equipment-free, high-throughput, flow-through microfluidic module versus traditional density gradient centrifugation.Cytotherapy. 2019 Feb;21(2):234-245. doi: 10.1016/j.jcyt.2018.12.004. Epub 2019 Jan 16. Cytotherapy. 2019. PMID: 30660490 Free PMC article.
-
Mechanisms of resistance to CAR T cell therapy.Nat Rev Clin Oncol. 2019 Jun;16(6):372-385. doi: 10.1038/s41571-019-0184-6. Nat Rev Clin Oncol. 2019. PMID: 30837712 Free PMC article. Review.
-
MDSC targeting with Gemtuzumab ozogamicin restores T cell immunity and immunotherapy against cancers.EBioMedicine. 2019 Sep;47:235-246. doi: 10.1016/j.ebiom.2019.08.025. Epub 2019 Aug 25. EBioMedicine. 2019. PMID: 31462392 Free PMC article.
References
-
- Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M, Shah NN, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–528. doi: 10.1016/S0140-6736(14)61403-3. - DOI - PMC - PubMed
-
- Kochenderfer JN, Yu Z, Frasheri D, Restifo NP, Rosenberg SA. Adoptive transfer of syngeneic T cells transduced with a chimeric antigen receptor that recognizes murine CD19 can eradicate lymphoma and normal B cells. Blood. 2010;116(19):3875–3886. doi: 10.1182/blood-2010-01-265041. - DOI - PMC - PubMed
-
- Brudno JN, Somerville RP, Shi V, Rose JJ, Halverson DC, Fowler DH, Gea-Banacloche JC, Pavletic SZ, Hickstein DD, Lu TL, et al. Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J Clin Oncol. 2016;34(10):1112–1121. doi: 10.1200/JCO.2015.64.5929. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

