VEGFR3 Modulates Vascular Permeability by Controlling VEGF/VEGFR2 Signaling
- PMID: 28298294
- PMCID: PMC6959003
- DOI: 10.1161/CIRCRESAHA.116.310477
VEGFR3 Modulates Vascular Permeability by Controlling VEGF/VEGFR2 Signaling
Abstract
Rationale: Vascular endothelial growth factor (VEGF) is the main driver of angiogenesis and vascular permeability via VEGF receptor 2 (VEGFR2), whereas lymphangiogenesis signals are transduced by VEGFC/D via VEGFR3. VEGFR3 also regulates sprouting angiogenesis and blood vessel growth, but to what extent VEGFR3 signaling controls blood vessel permeability remains unknown.
Objective: To investigate the role of VEGFR3 in the regulation of VEGF-induced vascular permeability.
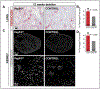
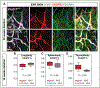
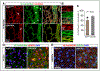
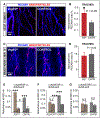
Methods and results: Long-term global Vegfr3 gene deletion in adult mice resulted in increased fibrinogen deposition in lungs and kidneys, indicating enhanced vascular leakage at the steady state. Short-term deletion of Vegfr3 in blood vascular endothelial cells increased baseline leakage in various tissues, as well as in tumors, and exacerbated vascular permeability in response to VEGF, administered via intradermal adenoviral delivery or through systemic injection of recombinant protein. VEGFR3 gene silencing upregulated VEGFR2 protein levels and phosphorylation in cultured endothelial cells. Consistent with elevated VEGFR2 activity, vascular endothelial cadherin showed reduced localization at endothelial cell-cell junctions in postnatal retinas after Vegfr3 deletion, or after VEGFR3 silencing in cultured endothelial cells. Furthermore, concurrent deletion of Vegfr2 prevented VEGF-induced excessive vascular leakage in mice lacking Vegfr3.
Conclusions: VEGFR3 limits VEGFR2 expression and VEGF/VEGFR2 pathway activity in quiescent and angiogenic blood vascular endothelial cells, thereby preventing excessive vascular permeability.
Keywords: VE-Cadherin; VEGF receptor regulation; blood vessels; vascular biology; vascular leakage.
© 2017 American Heart Association, Inc.
Figures



Similar articles
-
Molecular controls of lymphatic VEGFR3 signaling.Arterioscler Thromb Vasc Biol. 2015 Feb;35(2):421-9. doi: 10.1161/ATVBAHA.114.304881. Epub 2014 Dec 18. Arterioscler Thromb Vasc Biol. 2015. PMID: 25524775 Free PMC article.
-
Notch-dependent VEGFR3 upregulation allows angiogenesis without VEGF-VEGFR2 signalling.Nature. 2012 Mar 18;484(7392):110-4. doi: 10.1038/nature10908. Nature. 2012. PMID: 22426001
-
Erythropoietin maintains VE-cadherin expression and barrier function in experimental diabetic retinopathy via inhibiting VEGF/VEGFR2/Src signaling pathway.Life Sci. 2020 Oct 15;259:118273. doi: 10.1016/j.lfs.2020.118273. Epub 2020 Aug 12. Life Sci. 2020. PMID: 32800831
-
Two Birds, One Stone: Double Hits on Tumor Growth and Lymphangiogenesis by Targeting Vascular Endothelial Growth Factor Receptor 3.Cells. 2019 Mar 21;8(3):270. doi: 10.3390/cells8030270. Cells. 2019. PMID: 30901976 Free PMC article. Review.
-
Endothelial permeability and VE-cadherin: a wacky comradeship.Cell Adh Migr. 2013 Nov-Dec;7(6):455-61. doi: 10.4161/cam.27330. Epub 2013 Dec 5. Cell Adh Migr. 2013. PMID: 24430214 Free PMC article. Review.
Cited by
-
Formation of the glomerular microvasculature is regulated by VEGFR-3.Am J Physiol Renal Physiol. 2023 Jan 1;324(1):F91-F105. doi: 10.1152/ajprenal.00066.2022. Epub 2022 Nov 17. Am J Physiol Renal Physiol. 2023. PMID: 36395385 Free PMC article.
-
Mechanosensory entities and functionality of endothelial cells.Front Cell Dev Biol. 2024 Oct 23;12:1446452. doi: 10.3389/fcell.2024.1446452. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39507419 Free PMC article. Review.
-
Single-cell transcriptomics analysis of proliferative diabetic retinopathy fibrovascular membranes reveals AEBP1 as fibrogenesis modulator.JCI Insight. 2023 Nov 2;8(23):e172062. doi: 10.1172/jci.insight.172062. JCI Insight. 2023. PMID: 37917183 Free PMC article.
-
Is Wheat Glutenin Extract Intrinsically Allergenic? Evaluation Using a Novel Adjuvant-Free Mouse Model of Systemic Anaphylaxis.Int J Mol Sci. 2023 Dec 8;24(24):17247. doi: 10.3390/ijms242417247. Int J Mol Sci. 2023. PMID: 38139075 Free PMC article.
-
Sinusoidal and lymphatic vessel growth is controlled by reciprocal VEGF-C-CDH5 inhibition.Nat Cardiovasc Res. 2022 Nov;1(11):1006-1021. doi: 10.1038/s44161-022-00147-0. Epub 2022 Nov 11. Nat Cardiovasc Res. 2022. PMID: 36910472 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

